Inhibition of protein kinase CK2 suppresses angiogenesis and hematopoietic stem cell recruitment to retinal neovascularization sites
- PMID: 18612802
- PMCID: PMC2913688
- DOI: 10.1007/s11010-008-9831-4
Inhibition of protein kinase CK2 suppresses angiogenesis and hematopoietic stem cell recruitment to retinal neovascularization sites
Abstract
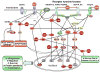
Ubiquitous protein kinase CK2 participates in a variety of key cellular functions. We have explored CK2 involvement in angiogenesis. As shown previously, CK2 inhibition reduced endothelial cell proliferation, survival and migration, tube formation, and secondary sprouting on Matrigel. Intraperitoneally administered CK2 inhibitors significantly reduced preretinal neovascularization in a mouse model of proliferative retinopathy. In this model, CK2 inhibitors had an additive effect with somatostatin analog, octreotide, resulting in marked dose reduction for the drug to achieve the same effect. CK2 inhibitors may thus emerge as potent future drugs aimed at inhibiting pathological angiogenesis. Immunostaining of the retina revealed predominant CK2 expression in astrocytes. In human diabetic retinas, mRNA levels of all CK2 subunits decreased, consistent with increased apoptosis. Importantly, a specific CK2 inhibitor prevented recruitment of bone marrow-derived hematopoietic stem cells to areas of retinal neovascularization. This may provide a novel mechanism of action of CK2 inhibitors on newly forming vessels.
Figures









References
-
- Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. - PubMed
-
- Unger GM, Davis AT, Slaton JW, et al. Protein kinase CK2 as regulator of cell survival: implications for cancer therapy. Curr Cancer Drug Targets. 2004;4:77–84. - PubMed
-
- Ahmad KA, Wang G, Slaton J, et al. Targeting CK2 for cancer therapy. Anticancer Drugs. 2005;16:1037–1043. - PubMed
-
- Sarno S, Salvi M, Battistutta R, Zanotti G, Pinna LA. Features and potentials of ATP-site directed CK2 inhibitors. Biochim Biophys Acta. 2005;1754:263–270. - PubMed
-
- Canton DA, Litchfield DW. The shape of things to come: an emerging role for protein kinase CK2 in the regulation of cell morphology and the cytoskeleton. Cell Signal. 2006;18:267–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

