Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence
- PMID: 18613151
- PMCID: PMC2695448
- DOI: 10.1002/hep.22351
Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence
Abstract
Capillarization precedes hepatic fibrosis. We hypothesize that capillarization of sinusoidal endothelial cells (SEC) is permissive for hepatic stellate cell (HSC) activation and therefore permissive for fibrosis. We examined whether freshly isolated SECs prevent activation of HSCs and promote reversion to quiescence, and whether this effect was lost in capillarization. HSCs were cultured alone or co-cultured with differentiated or capillarized SECs.
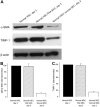
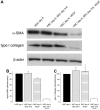
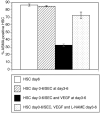
Results: Co-culture with freshly isolated SECs markedly decreased HSC activation after 3 days in culture, but co-culture with capillarized SEC had no effect. Inhibition of nitric oxide (NO) synthesis abolished SEC suppression of HSC activation. Activated HSCs reverted to quiescence when co-cultured with SEC plus vascular endothelial growth factor (VEGF) (that is, with SECs that maintained differentiation), but co-culture with capillarized SECs did not. Reversion of activated HSCs to quiescence in the presence of SECs plus VEGF was abolished by inhibition of NO synthesis. To establish whether there was indeed reversion, activated and quiescent HSCs were counted before and 3 days after adding freshly isolated SECs plus VEGF to activated HSCs, and proliferation was quantified in quiescent HSCs; the stoichiometry demonstrated reversion.
Conclusion: Differentiated SECs prevent HSC activation and promote reversion of activated HSCs to quiescence through VEGF-stimulated NO production. Capillarized SECs do not promote HSC quiescence, because of loss of VEGF-stimulated NO production.
Figures







References
-
- Schaffner F, Popper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239–242. - PubMed
-
- Horn T, Christoffersen P, Henriksen JH. Alcoholic liver injury: defenestration in noncirrhotic livers-a scanning electron microscopic study. HEPATOLOGY. 1987;7:77–82. - PubMed
-
- Mori T, Okanoue T, Sawa Y, Hori N, Ohta M, Kagawa K. Defenestration of the Sinusoidal Endothelial Cell in a Rat Model of Cirrhosis. HEPATOLOGY. 1993;17:891–897. - PubMed
-
- Horn T, Junge J, Christoffersen P. Early alcoholic liver injury: changes of the Disse space in acinar zone 3. Liver. 1985;5:301–310. - PubMed
-
- Mori T, Okanoue T, Kanaoka H, Sawa Y, Kashima K. Experimental study of the reversibility of sinusoidal capillarization. Alcohol and Alcoholism. 1994;29(Suppl 1):67–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
