A short-time scale colloidal system reveals early bacterial adhesion dynamics
- PMID: 18613749
- PMCID: PMC2443189
- DOI: 10.1371/journal.pbio.0060167
A short-time scale colloidal system reveals early bacterial adhesion dynamics
Abstract
The development of bacteria on abiotic surfaces has important public health and sanitary consequences. However, despite several decades of study of bacterial adhesion to inert surfaces, the biophysical mechanisms governing this process remain poorly understood, due, in particular, to the lack of methodologies covering the appropriate time scale. Using micrometric colloidal surface particles and flow cytometry analysis, we developed a rapid multiparametric approach to studying early events in adhesion of the bacterium Escherichia coli. This approach simultaneously describes the kinetics and amplitude of early steps in adhesion, changes in physicochemical surface properties within the first few seconds of adhesion, and the self-association state of attached and free-floating cells. Examination of the role of three well-characterized E. coli surface adhesion factors upon attachment to colloidal surfaces--curli fimbriae, F-conjugative pilus, and Ag43 adhesin--showed clear-cut differences in the very initial phases of surface colonization for cell-bearing surface structures, all known to promote biofilm development. Our multiparametric analysis revealed a correlation in the adhesion phase with cell-to-cell aggregation properties and demonstrated that this phenomenon amplified surface colonization once initial cell-surface attachment was achieved. Monitoring of real-time physico-chemical particle surface properties showed that surface-active molecules of bacterial origin quickly modified surface properties, providing new insight into the intricate relations connecting abiotic surface physicochemical properties and bacterial adhesion. Hence, the biophysical analytical method described here provides a new and relevant approach to quantitatively and kinetically investigating bacterial adhesion and biofilm development.
Conflict of interest statement
Figures
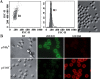


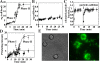


Comment in
-
Considering the first steps toward a stable and orderly way of bacterial life.PLoS Biol. 2008 Jul 15;6(7):e180. doi: 10.1371/journal.pbio.0060180. PLoS Biol. 2008. PMID: 18630990 Free PMC article.
References
-
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. - PubMed
-
- Katsikogianni M, Missirlis YF. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater. 2004;8:37–57. - PubMed
-
- Bos R, van der Mei HC, Busscher HJ. Physico-chemistry of initial microbial adhesive interactions–its mechanisms and methods for study. FEMS Microbiol Rev. 1999;23:179–230. - PubMed
-
- de Kerchove AJ, Elimelech M. Relevance of electrokinetic theory for “soft” particles to bacterial cells: implications for bacterial adhesion. Langmuir. 2005;21:6462–6472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

