Viral complementation allows HIV-1 replication without integration
- PMID: 18613957
- PMCID: PMC2474848
- DOI: 10.1186/1742-4690-5-60
Viral complementation allows HIV-1 replication without integration
Abstract
Background: The integration of HIV-1 DNA into cellular chromatin is required for high levels of viral gene expression and for the production of new virions. However, the majority of HIV-1 DNA remains unintegrated and is generally considered a replicative dead-end. A limited amount of early gene expression from unintegrated DNA has been reported, but viral replication does not proceed further in cells which contain only unintegrated DNA. Multiple infection of cells is common, and cells that are productively infected with an integrated provirus frequently also contain unintegrated HIV-1 DNA. Here we examine the influence of an integrated provirus on unintegrated HIV-1 DNA (uDNA).
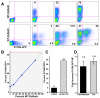
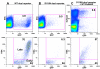
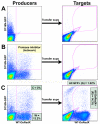
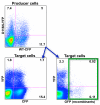
Results: We employed reporter viruses and quantitative real time PCR to examine gene expression and virus replication during coinfection with integrating and non-integrating HIV-1. Most cells which contained only uDNA displayed no detected expression from fluorescent reporter genes inserted into early (Rev-independent) and late (Rev-dependent) locations in the HIV-1 genome. Coinfection with an integrated provirus resulted in a several fold increase in the number of cells displaying uDNA early gene expression and efficiently drove uDNA into late gene expression. We found that coinfection generates virions which package and deliver uDNA-derived genomes into cells; in this way uDNA completes its replication cycle by viral complementation. uDNA-derived genomes undergo recombination with the integrated provirus-derived genomes during second round infection.
Conclusion: This novel mode of retroviral replication allows survival of viruses which would otherwise be lost because of a failure to integrate, amplifies the effective amount of cellular coinfection, increases the replicating HIV-1 gene pool, and enhances the opportunity for diversification through errors of polymerization and recombination.
Figures



References
-
- Brown PO. Integration. In: Coffin JM, Hughes SH, Varmus HE, editor. Retroviruses. Plainview NY: Cold Spring Harbor Laboratory Press; 1997. pp. 161–203. - PubMed
-
- Peterson S, Reid AP, Kim S, Siliciano RF. Treatment Implications of the Latent Reservoir for HIV-1. Advances in pharmacology (San Diego, Calif) 2007;55:411–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

