Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles
- PMID: 18614009
- PMCID: PMC3665268
- DOI: 10.1016/j.cell.2008.05.049
Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles
Abstract
Satellite cells reside beneath the basal lamina of skeletal muscle fibers and include cells that act as precursors for muscle growth and repair. Although they share a common anatomical localization and typically are considered a homogeneous population, satellite cells actually exhibit substantial heterogeneity. We used cell-surface marker expression to purify from the satellite cell pool a distinct population of skeletal muscle precursors (SMPs) that function as muscle stem cells. When engrafted into muscle of dystrophin-deficient mdx mice, purified SMPs contributed to up to 94% of myofibers, restoring dystrophin expression and significantly improving muscle histology and contractile function. Transplanted SMPs also entered the satellite cell compartment, renewing the endogenous stem cell pool and participating in subsequent rounds of injury repair. Together, these studies indicate the presence in adult skeletal muscle of prospectively isolatable muscle-forming stem cells and directly demonstrate the efficacy of myogenic stem cell transplant for treating muscle degenerative disease.
Figures
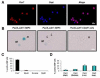

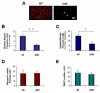

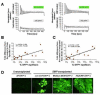

Comment in
-
Denominator problems in a muscle stem cell study?Cell. 2008 Dec 12;135(6):997-8; author reply 998-9. doi: 10.1016/j.cell.2008.11.033. Cell. 2008. PMID: 19070565 No abstract available.
References
-
- Bachrach E, Perez AL, Choi YH, Illigens BM, Jun SJ, del Nido P, McGowan FX, Li S, Flint A, Chamberlain J, Kunkel LM. Muscle engraftment of myogenic progenitor cells following intraarterial transplantation. Muscle Nerve. 2006;34:44–52. - PubMed
-
- Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev. 2006;16:525–532. - PubMed
-
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

