An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity
- PMID: 18614019
- PMCID: PMC4308735
- DOI: 10.1016/j.cell.2008.05.031
An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity
Abstract
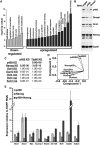
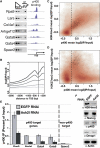
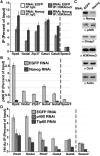
Proper regulation of chromatin structure is necessary for the maintenance of cell type-specific gene expression patterns. The embryonic stem cell (ESC) expression pattern governs self-renewal and pluripotency. Here, we present an RNAi screen in mouse ESCs of 1008 loci encoding chromatin proteins. We identified 68 proteins that exhibit diverse phenotypes upon knockdown (KD), including seven subunits of the Tip60-p400 complex. Phenotypic analyses revealed that Tip60-p400 is necessary to maintain characteristic features of ESCs. We show that p400 localization to the promoters of both silent and active genes is dependent upon histone H3 lysine 4 trimethylation (H3K4me3). Furthermore, the Tip60-p400 KD gene expression profile is enriched for developmental regulators and significantly overlaps with that of the transcription factor Nanog. Depletion of Nanog reduces p400 binding to target promoters without affecting H3K4me3 levels. Together, these data indicate that Tip60-p400 integrates signals from Nanog and H3K4me3 to regulate gene expression in ESCs.
Figures



References
-
- Baillat D, Hakimi MA, Naar AM, Shilatifard A, Cooch N, Shiekhattar R. Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell. 2005;123:265–276. - PubMed
-
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
-
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

