Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence
- PMID: 18614035
- PMCID: PMC2697387
- DOI: 10.1016/j.neuron.2008.04.031
Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence
Abstract
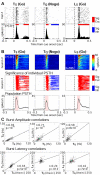
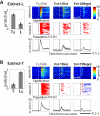
Both reward- and punishment-related stimuli are motivationally salient and attract the attention of animals. However, it remains unclear how motivational salience is processed in the brain. Here, we show that both reward- and punishment-predicting stimuli elicited robust bursting of many noncholinergic basal forebrain (BF) neurons in behaving rats. The same BF neurons also responded with similar bursting to primary reinforcement of both valences. Reinforcement responses were modulated by expectation, with surprising reinforcement eliciting stronger BF bursting. We further demonstrate that BF burst firing predicted successful detection of near-threshold stimuli. Together, our results point to the existence of a salience-encoding system independent of stimulus valence. We propose that the encoding of motivational salience by ensemble bursting of noncholinergic BF neurons may improve behavioral performance by affecting the activity of widespread cortical circuits and therefore represents a novel candidate mechanism for top-down attention.
Figures






Comment in
-
Noncholinergic neurons in the basal forebrain: often neglected but motivationally salient.Neuron. 2008 Jul 10;59(1):6-8. doi: 10.1016/j.neuron.2008.06.017. Neuron. 2008. PMID: 18614024
References
-
- Alonso A, Khateb A, Fort P, Jones BE, Muhlethaler M. Differential oscillatory properties of cholinergic and noncholinergic nucleus basalis neurons in guinea pig brain slice. Eur J Neurosci. 1996;8:169–182. - PubMed
-
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. - PubMed
-
- Burk JA, Sarter M. Dissociation between the attentional functions mediated via basal forebrain cholinergic and GABAergic neurons. Neuroscience. 2001;105:899–909. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

