Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial
- PMID: 18614482
- PMCID: PMC2483870
- DOI: 10.1136/bmj.a339
Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial
Abstract
Objective: To compare routine replacement of intravenous peripheral catheters with replacement only when clinically indicated.
Design: Randomised controlled trial.
Setting: Tertiary hospital in Australia.
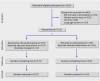
Participants: 755 medical and surgical patients: 379 allocated to catheter replacement only when clinically indicated and 376 allocated to routine care of catheter (control group).
Main outcome measure: A composite measure of catheter failure resulting from phlebitis or infiltration.
Results: Catheters were removed because of phlebitis or infiltration from 123 of 376 (33%) patients in the control group compared with 143 of 379 (38%) patients in the intervention group; the difference was not significant (relative risk 1.15, 95% confidence interval 0.95 to 1.40). When the analysis was based on failure per 1000 device days (number of failures divided by number of days catheterised, divided by 1000), no difference could be detected between the groups (relative risk 0.98, 0.78 to 1.24). Infusion related costs were higher in the control group (mean $A41.02; pound19.71; euro24.80; $38.55) than intervention group ($A36.40). The rate of phlebitis in both groups was low (4% in intervention group, 3% in control group).
Conclusion: Replacing peripheral intravenous catheters when clinically indicated has no effect on the incidence of failure, based on a composite measure of phlebitis or infiltration. Larger trials are needed to test this finding using phlebitis alone as a more clinically meaningful outcome.
Registration number: Australian New Zealand Clinical Trials Registry ACTRN12605000147684.
Conflict of interest statement
Competing interests: None declared.
Comment in
-
Improving the safety of peripheral intravenous catheters.BMJ. 2008 Jul 8;337(7662):a630. doi: 10.1136/bmj.a630. BMJ. 2008. PMID: 18614483 Free PMC article.
-
Clinically indicated and routine replacement of peripheral intravenous catheters did not differ for catheter failure.Evid Based Nurs. 2009 Jan;12(1):19. doi: 10.1136/ebn.12.1.19. Evid Based Nurs. 2009. PMID: 19103837 No abstract available.
References
-
- Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med 1998;158:151-6. - PubMed
-
- Tripepi-Bova KA, Woods KD, Loach MC. A comparison of transparent polyurethane and dry gauze dressings for peripheral IV catheter sites: rates of phlebitis, infiltration, and dislodgment by patients. Am J Crit Care 1997;6:377-81. - PubMed
-
- Chee S, Tan W. Reducing infusion phlebitis in Singapore hospitals using extended life end-line filters. J Infus Nurs 2002;25:95-104. - PubMed
-
- Tager IB, Ginsberg MB, Ellis SE, Walsh NE, Dupont I, Simchen E, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol 1983;118:839-51. - PubMed
-
- Martinez JA, Fernandez P, Rodriguez E, Sobrino J, Torres M, Nubiola A, et al. Intravenous cannulae: complications arising from their use and analysis of their predisposing factors. Med Clin (Barc) 1994;103:89-93. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical