Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA)
- PMID: 18614531
- PMCID: PMC2533091
- DOI: 10.1074/jbc.M804146200
Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA)
Abstract
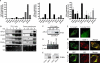
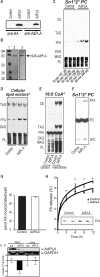
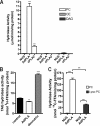
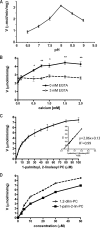
Phospholipases A(2) (PLA(2)s) catalyze hydrolysis of fatty acids from the sn-2 position of phospholipids. Here we report the identification and characterization of a membrane-associated intracellular calcium-dependent, adipose-specific PLA(2) that we named AdPLA (adipose-specific phospholipase A(2)). We found that AdPLA was highly expressed specifically in white adipose tissue and was induced during preadipocyte differentiation into adipocytes. Clearance of AdPLA by immunoprecipitation significantly decreased PLA activity in white adipose tissue lysates but had no effect on liver lysates, where expression was hardly detectable. In characterizing AdPLA, we employed radiochemical assays with TLC analysis of the enzyme activity of lysates from COS-7 cells overexpressing AdPLA. For kinetic studies, we produced purified recombinant AdPLA for use in a lipoxidase-coupled spectrophotometric assay. AdPLA generated free fatty acid and lysophospholipid from phosphatidylcholine with a preference for hydrolysis at the sn-2 position. Although we found low but detectable lysophospholipase activity, AdPLA showed no significant activity against a variety of other lipid substrates. Calcium was found to activate AdPLA but was not essential for activity. Studies with known phospholipase inhibitors, including bromoenolactone, methyl arachidonyl fluorophosphate, AACOCF(3), 7,7-dimethyl-5,8-eicosadienoic acid, and thioetheramide, supported that AdPLA is a phospholipase. Mutational studies showed that His-23 and Cys-113 are critical for activity of AdPLA and suggested that AdPLA is likely a His/Cys PLA(2). Overall, although AdPLA is similar to other histidine phospholipases in pH and calcium dependence, AdPLA showed different characteristics in many regards, including predicted catalytic mechanism. AdPLA may therefore represent the first member of a new group of PLA(2)s, group XVI.
Figures
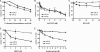

References
-
- Schaloske, R. H., and Dennis, E. A. (2006) Biochim. Biophys. Acta 1761 1246-1259 - PubMed
-
- Aubert, J., Saint-Marc, P., Belmonte, N., Dani, C., Negrel, R., and Ailhaud, G. (2000) Mol. Cell. Endocrinol. 160 149-156 - PubMed
-
- Fajas, L., Miard, S., Briggs, M. R., and Auwerx, J. (2003) J. Lipid Res. 44 1652-1659 - PubMed
-
- Forman, B. M., Tontonoz, P., Chen, J., Brun, R. P., Spiegelman, B. M., and Evans, R. M. (1995) Cell 83 803-812 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

