Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification
- PMID: 18614539
- PMCID: PMC2533093
- DOI: 10.1074/jbc.M800882200
Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification
Abstract
Rho GTPases (20 human members) comprise a major branch of the Ras superfamily of small GTPases, and aberrant Rho GTPase function has been implicated in oncogenesis and other human diseases. Although many of our current concepts of Rho GTPases are based on the three classical members (RhoA, Rac1, and Cdc42), recent studies have revealed the diversity of biological functions mediated by other family members. A key basis for the functional diversity of Rho GTPases is their association with distinct subcellular compartments, which is dictated in part by three posttranslational modifications signaled by their carboxyl-terminal CAAX (where C represents cysteine, A is an aliphatic amino acid, and X is a terminal amino acid) tetrapeptide motifs. CAAX motifs are substrates for the prenyltransferase-catalyzed addition of either farnesyl or geranylgeranyl isoprenoid lipids, Rce1-catalyzed endoproteolytic cleavage of the AAX amino acids, and Icmt-catalyzed carboxyl methylation of the isoprenylcysteine. We utilized pharmacologic, biochemical, and genetic approaches to determine the sequence requirements and roles of CAAX signal modifications in dictating the subcellular locations and functions of the Rho GTPase family. Although the classical Rho GTPases are modified by geranylgeranylation, we found that a majority of the other Rho GTPases are substrates for farnesyltransferase. We found that the membrane association and/or function of Rho GTPases are differentially dependent on Rce1- and Icmt-mediated modifications. Our results further delineate the sequence requirements for prenyltransferase specificity and functional roles for protein prenylation in Rho GTPase function. We conclude that a majority of Rho GTPases are targets for pharmacologic inhibitors of farnesyltransferase, Rce1, and Icmt.
Figures
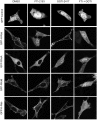
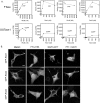
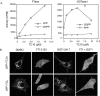

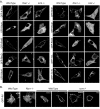

References
-
- Wennerberg, K., Rossman, K. L., and Der, C. J. (2005) J. Cell Sci. 118 843–846 - PubMed
-
- Wennerberg, K., and Der, C. J. (2004) J. Cell Sci. 117 1301–1312 - PubMed
-
- Schmidt, A., and Hall, A. (2002) Genes Dev. 16 1587–1609 - PubMed
-
- Bernards, A., and Settleman, J. (2004) Trends Cell Biol. 14 377–385 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

