The NY-1 hantavirus Gn cytoplasmic tail coprecipitates TRAF3 and inhibits cellular interferon responses by disrupting TBK1-TRAF3 complex formation
- PMID: 18614628
- PMCID: PMC2546897
- DOI: 10.1128/JVI.00290-08
The NY-1 hantavirus Gn cytoplasmic tail coprecipitates TRAF3 and inhibits cellular interferon responses by disrupting TBK1-TRAF3 complex formation
Abstract
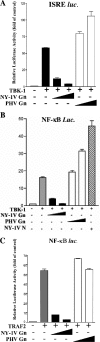
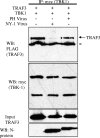
Pathogenic hantaviruses replicate within human endothelial cells and cause two diseases, hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. In order to replicate in endothelial cells pathogenic hantaviruses inhibit the early induction of beta interferon (IFN-beta). Expression of the cytoplasmic tail of the pathogenic NY-1 hantavirus Gn protein is sufficient to inhibit RIG-I- and TBK1-directed IFN responses. The formation of TBK1-TRAF3 complexes directs IRF-3 phosphorylation, and both IRF-3 and NF-kappaB activation are required for transcription from the IFN-beta promoter. Here we report that the NY-1 virus (NY-1V) Gn tail inhibits both TBK1-directed NF-kappaB activation and TBK1-directed transcription from promoters containing IFN-stimulated response elements. The NY-1V Gn tail coprecipitated TRAF3 from cellular lysates, and analysis of TRAF3 deletion mutants demonstrated that the TRAF3 N terminus is sufficient for interacting with the NY-1V Gn tail. In contrast, the Gn tail of the nonpathogenic hantavirus Prospect Hill virus (PHV) failed to coprecipitate TRAF3 or inhibit NF-kappaB or IFN-beta transcriptional responses. Further, expression of the NY-1V Gn tail blocked TBK1 coprecipitation of TRAF3 and infection by NY-1V, but not PHV, blocked the formation of TBK1-TRAF3 complexes. These findings indicate that the NY-1V Gn cytoplasmic tail forms a complex with TRAF3 which disrupts the formation of TBK1-TRAF3 complexes and downstream signaling responses required for IFN-beta transcription.
Figures



References
-
- Blakqori, G., S. Delhaye, M. Habjan, C. D. Blair, I. Sanchez-Vargas, K. E. Olson, G. Attarzadeh-Yazdi, R. Fragkoudis, A. Kohl, U. Kalinke, S. Weiss, T. Michiels, P. Staeheli, and F. Weber. 2007. La Crosse bunyavirus nonstructural protein NSs serves to suppress the type I interferon system of mammalian hosts. J. Virol. 814991-4999. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

