Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection
- PMID: 18614631
- PMCID: PMC2546878
- DOI: 10.1128/JVI.01041-08
Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection
Abstract
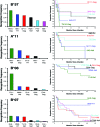
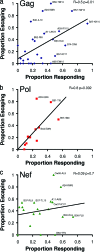
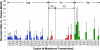
During acute human immunodeficiency virus type 1 (HIV-1) infection, early host cellular immune responses drive viral evolution. The rates and extent of these mutations, however, remain incompletely characterized. In a cohort of 98 individuals newly infected with HIV-1 subtype B, we longitudinally characterized the rates and extent of HLA-mediated escape and reversion in Gag, Pol, and Nef using a rational definition of HLA-attributable mutation based on the analysis of a large independent subtype B data set. We demonstrate rapid and dramatic HIV evolution in response to immune pressures that in general reflect established cytotoxic T-lymphocyte (CTL) response hierarchies in early infection. On a population level, HLA-driven evolution was observed in approximately 80% of published CTL epitopes. Five of the 10 most rapidly evolving epitopes were restricted by protective HLA alleles (HLA-B*13/B*51/B*57/B*5801; P = 0.01), supporting the importance of a strong early CTL response in HIV control. Consistent with known fitness costs of escape, B*57-associated mutations in Gag were among the most rapidly reverting positions upon transmission to non-B*57-expressing individuals, whereas many other HLA-associated polymorphisms displayed slow or negligible reversion. Overall, an estimated minimum of 30% of observed substitutions in Gag/Pol and 60% in Nef were attributable to HLA-associated escape and reversion events. Results underscore the dominant role of immune pressures in driving early within-host HIV evolution. Dramatic differences in escape and reversion rates across codons, genes, and HLA restrictions are observed, highlighting the complexity of viral adaptation to the host immune response.
Figures


References
-
- Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 772081-2092. - PMC - PubMed
-
- Allen, T. M., M. Altfeld, S. C. Geer, E. T. Kalife, C. Moore, M. O'Sullivan, K. I. Desouza, M. E. Feeney, R. L. Eldridge, E. L. Maier, D. E. Kaufmann, M. P. Lahaie, L. Reyor, G. Tanzi, M. N. Johnston, C. Brander, R. Draenert, J. K. Rockstroh, H. Jessen, E. S. Rosenberg, S. A. Mallal, and B. D. Walker. 2005. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J. Virol. 7913239-13249. - PMC - PubMed
-
- Allen, T. M., X. G. Yu, E. T. Kalife, L. L. Reyor, M. Lichterfeld, M. John, M. Cheng, R. L. Allgaier, S. Mui, N. Frahm, G. Alter, N. V. Brown, M. N. Johnston, E. S. Rosenberg, S. A. Mallal, C. Brander, B. D. Walker, and M. Altfeld. 2005. De novo generation of escape variant-specific CD8+ T-cell responses following cytotoxic T-lymphocyte escape in chronic human immunodeficiency virus type 1 infection. J. Virol. 7912952-12960. - PMC - PubMed
-
- Altfeld, M., M. M. Addo, E. S. Rosenberg, F. M. Hecht, P. K. Lee, M. Vogel, X. G. Yu, R. Draenert, M. N. Johnston, D. Strick, T. M. Allen, M. E. Feeney, J. O. Kahn, R. P. Sekaly, J. A. Levy, J. K. Rockstroh, P. J. Goulder, and B. D. Walker. 2003. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 172581-2591. - PubMed
-
- Altfeld, M., M. M. Addo, R. Shankarappa, P. K. Lee, T. M. Allen, X. G. Yu, A. Rathod, J. Harlow, K. O'Sullivan, M. N. Johnston, P. J. Goulder, J. I. Mullins, E. S. Rosenberg, C. Brander, B. Korber, and B. D. Walker. 2003. Enhanced detection of human immunodeficiency virus type 1-specific T-cell responses to highly variable regions by using peptides based on autologous virus sequences. J. Virol. 777330-7340. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

