Influenza A virus matrix protein 1-specific human CD8+ T-cell response induced in trivalent inactivated vaccine recipients
- PMID: 18614638
- PMCID: PMC2546892
- DOI: 10.1128/JVI.01047-08
Influenza A virus matrix protein 1-specific human CD8+ T-cell response induced in trivalent inactivated vaccine recipients
Abstract
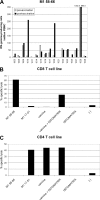
Among 17 HLA-A2-positive healthy adults, CD8+ T-cell responses against an HLA-A2-restricted matrix protein 1 (M1) epitope increased after immunization with trivalent inactivated influenza vaccine (TIV) in two individuals. The presence of M1 in TIV was confirmed by Western blotting. T-cell cytotoxicity assays showed that TIV is processed and the epitope is presented by antigen-presenting cells to an M1 epitope-specific CD8+ T-cell line for specific lysis. These data show that TIV, which is formulated to contain surface glycoproteins to induce serotype-specific antibody responses, also contains M1, capable of inducing subtype cross-reactive CD8+ T-cell responses in some vaccinees.
Figures

References
-
- Beyer, W. E., A. M. Palache, J. C. de Jong, and A. D. Osterhaus. 2002. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine 201340-1353. - PubMed
-
- Centers for Disease Control and Prevention. 26 June 2008, posting date. 2007-2008 flu season. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/flu/about/qa/season/index.htm.
-
- Ennis, F. A., J. Cruz, J. Jameson, M. Klein, D. Burt, and J. Thipphawong. 1999. Augmentation of human influenza A virus-specific cytotoxic T lymphocyte memory by influenza vaccine and adjuvanted carriers (ISCOMS). Virology 259256-261. - PubMed
-
- Epstein, S. L. 2006. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J. Infect. Dis. 19349-53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

