GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism
- PMID: 18614712
- PMCID: PMC2528133
- DOI: 10.1104/pp.108.121400
GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism
Abstract
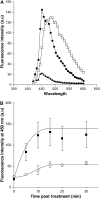
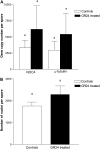
Arbuscular mycorrhizal (AM) fungi are obligate biotrophs that participate in a highly beneficial root symbiosis with 80% of land plants. Strigolactones are trace molecules in plant root exudates that are perceived by AM fungi at subnanomolar concentrations. Within just a few hours, they were shown to stimulate fungal mitochondria, spore germination, and branching of germinating hyphae. In this study we show that treatment of Gigaspora rosea with a strigolactone analog (GR24) causes a rapid increase in the NADH concentration, the NADH dehydrogenase activity, and the ATP content of the fungal cell. This fully and rapidly (within minutes) activated oxidative metabolism does not require new gene expression. Up-regulation of the genes involved in mitochondrial metabolism and hyphal growth, and stimulation of the fungal mitotic activity, take place several days after this initial boost to the cellular energy of the fungus. Such a rapid and powerful action of GR24 on G. rosea cells suggests that strigolactones are important plant signals involved in switching AM fungi toward full germination and a presymbiotic state.
Figures



Comment in
-
Role of mitochondria in the response of arbuscular mycorrhizal fungi to strigolactones.Plant Signal Behav. 2009 Jan;4(1):75-7. doi: 10.4161/psb.4.1.7419. Plant Signal Behav. 2009. PMID: 19704715 Free PMC article.
References
-
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435 824–827 - PubMed
-
- Andersson H, Baechi T, Hoechl M, Richter C (1998) Autofluorescence of living cells. J Microsc 191 1–7 - PubMed
-
- Babiker AGT, Ejeta G, Butler LG, Woodson WR (1993) Ethylene biosynthesis and strigol-induced germination of Striga asiatica. Physiol Plant 88 359–365
-
- Bachman NJ, Wu W, Schmidt TR, Grossman LI, Lomax MI (1999) The 5′ region of the COX4 gene contains a novel overlapping gene, NOC4. Mamm Genome 10 506–512 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

