Nephrin mutations can cause childhood-onset steroid-resistant nephrotic syndrome
- PMID: 18614772
- PMCID: PMC2551572
- DOI: 10.1681/ASN.2008010059
Nephrin mutations can cause childhood-onset steroid-resistant nephrotic syndrome
Abstract
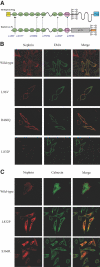
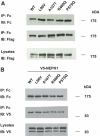
Classically, infants with mutations in NPHS1, which encodes nephrin, present with nephrotic syndrome within the first 3 mo of life (congenital nephrotic syndrome of the Finnish-type), and children with mutations in NPHS2, which encodes podocin, present later with steroid-resistant nephrotic syndrome. Recently, however, NPHS2 mutations have been identified in children with congenital nephrotic syndrome. Whether NPHS1 mutations similarly account for some cases of childhood steroid-resistant nephrotic syndrome is unknown. In this study, 160 patients who belonged to 142 unrelated families and presented with nephrotic syndrome at least 3 mo after birth were screened for NPHS1 variants once mutations in NPHS2 had been excluded. Compound heterozygous NPHS1 mutations were identified in one familial case and nine sporadic cases. Mutations included protein-truncating nonsense and frameshift mutations, as well as splice-site and missense variants. Mutations were classified as "severe" or "mild" using prediction algorithms and functional assays. Most missense variants trafficked normally to the plasma membrane and maintained the ability to form nephrin homodimers and to heterodimerize with NEPH1, suggesting retained function. The presence of at least one "mild" mutation in these patients likely explains the later onset and milder course of disease. These results broaden the spectrum of renal disease related to nephrin mutations.
Figures

Comment in
-
Yet more ways to skin a cat: nephrin mutations outside the neonatal period.J Am Soc Nephrol. 2008 Oct;19(10):1837-8. doi: 10.1681/ASN.2008070753. Epub 2008 Aug 27. J Am Soc Nephrol. 2008. PMID: 18753252 No abstract available.
References
-
- Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 - PubMed
-
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1988 - PubMed
-
- Gigante M, Monno F, Roberto R, Laforgia N, Assael MB, Livolti S, Caringella A, La Manna A, Masella L, Iolascon A: Congenital nephrotic syndrome of the Finnish type in Italy: A molecular approach. J Nephrol 15: 696–702, 2002 - PubMed
-
- Koziell A, Grech V, Hussain S, Lee G, Lenkkeri U, Tryggvason K, Scambler P: Genotype/phenotype correlations of NPHS1 and NPHS2 mutations in nephrotic syndrome advocate a functional inter-relationship in glomerular filtration. Hum Mol Genet 11: 379–388, 2002 - PubMed
-
- Sako M, Nakanishi K, Obana M, Yata N, Hoshii S, Takahashi S, Wada N, Takahashi Y, Kaku Y, Satomura K, Ikeda M, Honda M, Iijima K, Yoshikawa N: Analysis of NPHS1, NPHS2, ACTN4, and WT1 in Japanese patients with congenital nephrotic syndrome. Kidney Int 67: 1248–1255, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

