Effect of HAART on incident cancer and noncancer AIDS events among male HIV seroconverters
- PMID: 18614916
- PMCID: PMC2805176
- DOI: 10.1097/QAI.0b013e31817dc42b
Effect of HAART on incident cancer and noncancer AIDS events among male HIV seroconverters
Abstract
Objective: To explore the impact of highly active antiretroviral therapy (HAART) on the prevention of AIDS-defining cancers relative to other AIDS-defining events.
Design: Prospective cohort study using 2,121 HIV+ male seroconverters (median age: 28 years, 51% white/non-Hispanic) in the Tri-Service AIDS Clinical Consortium (n = 1694) and the Multicenter AIDS Cohort Study (n = 427).
Methods: Poisson regression models, with calendar periods to represent antiretroviral therapy, were extended to analyze first incident AIDS-defining cancers and other first AIDS-defining events as competing risks.
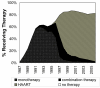
Results: Eighty-one AIDS-defining cancers (64 Kaposi sarcomas; 17 non-Hodgkin lymphomas) and 343 other AIDS events occurred during 14,483 person-years in 1990-2006. The rate ratio of AIDS-defining cancers during the HAART calendar period was 0.26 (95% confidence limits: 0.15, 0.46) and of other AIDS-defining events was 0.28 (95% confidence limits: 0.21, 0.36) compared with the monotherapy/combination therapy calendar period, adjusting for age, infection duration, race, and cohort. The association of HAART with decreased AIDS incidence seemed to be equal (interaction ratio = 0.95 (95% confidence limits: 0.51, 1.74) for AIDS-defining cancers and other AIDS-defining events.
Conclusions: In human immunodeficiency virus-infected men, HAART seems equally protective against first AIDS-defining cancers and other first AIDS-defining events.
Figures
References
-
- Cameron DW, Heath-Chiozzi M, Danner S, et al. The Advanced HIV Disease Ritonavir Study Group Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. Lancet. 1998;351(9102):543–549. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337(11):725–733. - PubMed
-
- Detels R, Munoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA. 1998;280(17):1497–1503. - PubMed
-
- Palella FJ, Jr., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. - PubMed
-
- Cole SR, Hernan MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158(7):687–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UO1-AI-35042/AI/NIAID NIH HHS/United States
- UO1-AI-37984/AI/NIAID NIH HHS/United States
- UO1-AI-35040/AI/NIAID NIH HHS/United States
- M01 RR000722/RR/NCRR NIH HHS/United States
- U01 AI035041/AI/NIAID NIH HHS/United States
- U01 AI035043/AI/NIAID NIH HHS/United States
- UO1-AI-35039/AI/NIAID NIH HHS/United States
- U01 AI035040/AI/NIAID NIH HHS/United States
- 5-MO1-RR-00722/RR/NCRR NIH HHS/United States
- U01 AI035039/AI/NIAID NIH HHS/United States
- HU0001-05-2-0011/AI/NIAID NIH HHS/United States
- U01 AI035042/AI/NIAID NIH HHS/United States
- U01 AI037984/AI/NIAID NIH HHS/United States
- U01 DK066116/DK/NIDDK NIH HHS/United States
- U01 AI037613/AI/NIAID NIH HHS/United States
- UO1-AI-35041/AI/NIAID NIH HHS/United States
- UO1-AI-35043/AI/NIAID NIH HHS/United States
- UO1-AI-37613/AI/NIAID NIH HHS/United States
- T32 CA009314/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical