Overexpression of IL-1alpha in skin differentially modulates the immune response to scarification with vaccinia virus
- PMID: 18615110
- PMCID: PMC2841968
- DOI: 10.1038/jid.2008.191
Overexpression of IL-1alpha in skin differentially modulates the immune response to scarification with vaccinia virus
Abstract
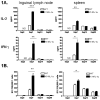
Transepidermal inoculation of vaccinia virus (VV), or scarification, has been used effectively for the induction of specific and long-lasting immunity to smallpox and is superior to other routes of immunization. Scarification of individuals with atopic skin disease or immune deficiency, however, can lead to persistent viral replication and result in significant morbidity and mortality. These effects of scarification presumably reflect the unique immunological properties of skin and the immune cells resident in, or recruited to, the site of inoculation. To explore these phenomena, we utilized transgenic mice engineered to overexpress IL-1alpha, a critical mediator of cutaneous inflammation, in the epidermis. Following scarification with VV, both transgenic and wild-type mice develop local pox. At high doses of VV, IL-1alpha transgenic mice recruited immune cells to the inoculation site more rapidly and demonstrated enhanced T-cell and humoral immune responses. At limiting doses, however, IL-1alpha transgenic mice could effectively control virus replication without formation of pox lesions or activation of a memory response. This study suggests that IL-1 might be useful as an adjuvant to enhance antiviral immunity and promote safer vaccination strategies; however, understanding the balance of IL-1 effects on innate and adaptive immune functions will be critical to achieve optimal results.
Conflict of interest statement
Figures


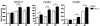

References
-
- Alcami A. Viral mimicry of cytokines, chemokines and their receptors. Nat Rev Immunol. 2003;3:36–50. - PubMed
-
- Alcami A, Smith GL. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. - PubMed
-
- Alcami A, Symons JA, Collins PD, Williams TJ, Smith GL. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J Immunol. 1998;160:624–633. - PubMed
-
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral research. 2003;58:101–114. - PubMed
-
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

