Direct functionalization of nitrogen heterocycles via Rh-catalyzed C-H bond activation
- PMID: 18616300
- PMCID: PMC2610463
- DOI: 10.1021/ar800042p
Direct functionalization of nitrogen heterocycles via Rh-catalyzed C-H bond activation
Abstract
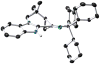
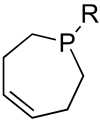
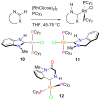
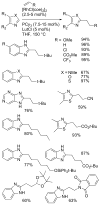
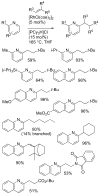
[Reaction: see text]. Nitrogen heterocycles are present in many compounds of enormous practical importance, ranging from pharmaceutical agents and biological probes to electroactive materials. Direct functionalization of nitrogen heterocycles through C-H bond activation constitutes a powerful means of regioselectively introducing a variety of substituents with diverse functional groups onto the heterocycle scaffold. Working together, our two groups have developed a family of Rh-catalyzed heterocycle alkylation and arylation reactions that are notable for their high level of functional-group compatibility. This Account describes our work in this area, emphasizing the relevant mechanistic insights that enabled synthetic advances and distinguished the resulting transformations from other methods. We initially discovered an intramolecular Rh-catalyzed C-2 alkylation of azoles by alkenyl groups. That reaction provided access to a number of di-, tri-, and tetracyclic azole derivatives. We then developed conditions that exploited microwave heating to expedite these reactions. While investigating the mechanism of this transformation, we discovered that a novel substrate-derived Rh- N-heterocyclic carbene (NHC) complex was involved as an intermediate. We then synthesized analogous Rh-NHC complexes directly by treating precursors to the intermediate [RhCl(PCy 3)2] with N-methylbenzimidazole, 3-methyl-3,4-dihydroquinazoline, and 1-methyl-1,4-benzodiazepine-2-one. Extensive kinetic analysis and DFT calculations supported a mechanism for carbene formation in which the catalytically active RhCl(PCy 3) 2 fragment coordinates to the heterocycle before intramolecular activation of the C-H bond occurs. The resulting Rh-H intermediate ultimately tautomerizes to the observed carbene complex. With this mechanistic information and the discovery that acid cocatalysts accelerate the alkylation, we developed conditions that efficiently and intermolecularly alkylate a variety of heterocycles, including azoles, azolines, dihydroquinazolines, pyridines, and quinolines, with a wide range of functionalized olefins. We demonstrated the utility of this methodology in the synthesis of natural products, drug candidates, and other biologically active molecules. In addition, we developed conditions to directly arylate these heterocycles with aryl halides. Our initial conditions that used PCy 3 as a ligand were successful only for aryl iodides. However, efforts designed to avoid catalyst decomposition led to the development of ligands based on 9-phosphabicyclo[4.2.1]nonane (phoban) that also facilitated the coupling of aryl bromides. We then replicated the unique coordination environment, stability, and catalytic activity of this complex using the much simpler tetrahydrophosphepine ligands and developed conditions that coupled aryl bromides bearing diverse functional groups without the use of a glovebox or purified reagents. With further mechanistic inquiry, we anticipate that researchers will better understand the details of the aforementioned Rh-catalyzed C-H bond functionalization reactions, resulting in the design of more efficient and robust catalysts, expanded substrate scope, and new transformations.
Figures



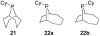











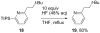
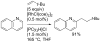
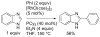
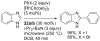






References
-
- Dyker G. Transition Metal Catalyzed Coupling Reactions Under C-H Activation. Angew Chem Int Ed. 1999;38:1698. - PubMed
- Kakiuchi F, Murai S. Activation of CH Bonds: Catalytic Reactions. Top Curr Chem. 1999;3:47.
- Miura M, Nomura M. Direct Arylation via Cleavage of Activated and Unactivated C-H Bonds. Top Curr Chem. 2002;219:212.
- Campeau L-C, Fagnou K. Palladium-Catalyzed Direct Arylation of Simple Arenes in Synthesis of Biaryl Molecules. Chem Commun. 2006:1253. - PubMed
- Daugulis O, Zaitsev VG, Shabashov D, Pham Q-N, Lazareva A. Regioselective Functionalization of Unreactive Carbon-Hydrogen Bonds. Synlett. 2006;20:3382.
- Alberico D, Scott ME, Lautens M. Aryl-aryl bond formation by transition-metal-catalyzed direct arylation. Chem Rev. 2007;107:174–238. - PubMed
-
- Diederich F, Stang PJ, editors. Metal-catalyzed Cross-coupling Reactions. Wiley-VCH; New York: 1998.
-
- Crabtree RH. Alkane C-H Activation and Functionalization with Homogeneous Transition Metal Catalysts: a Century of Progress-a New Millennium in Prospect. Dalton. 2001:2437–2450.
- Bercaw JE, Labinger JA. Understanding and Exploiting C-H Bond Activation. Nature. 2002;417:507–514. - PubMed
- Bergman RG. Organometallic Chemistry: C-H Activation. Nature. 2007;446:391–393. - PubMed
-
- Daugulis O, Zaitsev VG. Anilide Ortho-Arylation by Using C-H Activation Methodology. Angew Chem Int Ed. 2005;4:4046. - PubMed
- Kalyani D, Deprez NR, Desai LV, Sanford MS. Oxidative C-H Activation/C-C Bond Forming Reactions: Synthetic Scope and Mechanistic Insights. J Am Chem Soc. 2005;127:7330. - PubMed
- Kakiuchi F, Murai S. Catalytic C-H/Olefin Coupling. Acc Chem Res. 2002;35:826–834. - PubMed
-
- Cho J-Y, Iverson CN, Smith MR. Steric and Chelate Directing Effects in Aromatic Borylation. J Am Chem Soc. 2000;122:12868.
- Ishiyama T, Takagi J, Kousaku I, Miyaura N, Anastasi NR, Hartwig JF. Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate. J Am Chem Soc. 2002;124:390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

