Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme
- PMID: 18616418
- PMCID: PMC2832832
- DOI: 10.1517/13543784.17.8.1225
Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme
Abstract
Background: Glioblastoma multiforme (GBM), a highly invasive and vascular cancer, responds poorly to conventional cytotoxic therapy. Integrins, widely expressed in GBM and tumor vasculature, mediate cell survival, migration and angiogenesis. Cilengitide is a potent alphavbeta3 and alphavbeta5 integrin inhibitor.
Objective: To summarize the preclinical and clinical experience with cilengitide for GBM.
Methods: Preclinical studies and clinical trials evaluating cilengitide for GBM were reviewed.
Results/conclusions: Cilengitide is active and synergizes with external beam radiotherapy in preclinical GBM models. In clinical trials for recurrent GBM, single-agent cilengitide has antitumor benefits and minimal toxicity. Among newly diagnosed GBM patients, single-arm studies incorporating cilengitide into standard external beam radiotherapy/temozolomide have shown encouraging activity with no increased toxicity and have led to a planned randomized Phase III trial.
Conflict of interest statement
Figures

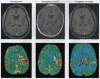
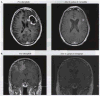
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. - PubMed
-
- Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–9. - PubMed
-
- Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
