Communication between the synapse and the nucleus in neuronal development, plasticity, and disease
- PMID: 18616423
- PMCID: PMC2709812
- DOI: 10.1146/annurev.cellbio.24.110707.175235
Communication between the synapse and the nucleus in neuronal development, plasticity, and disease
Abstract
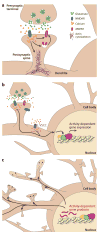
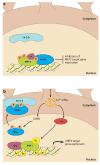
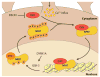
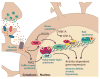
Sensory experience is critical for the proper development and plasticity of the brain throughout life. Successful adaptation to the environment is necessary for the survival of an organism, and this process requires the translation of specific sensory stimuli into changes in the structure and function of relevant neural circuits. Sensory-evoked activity drives synaptic input onto neurons within these behavioral circuits, initiating membrane depolarization and calcium influx into the cytoplasm. Calcium signaling triggers the molecular mechanisms underlying neuronal adaptation, including the activity-dependent transcriptional programs that drive the synthesis of the effector molecules required for long-term changes in neuronal function. Insight into the signaling pathways between the synapse and the nucleus that translate specific stimuli into altered patterns of connectivity within a circuit provides clues as to how activity-dependent programs of gene expression are coordinated and how disruptions in this process may contribute to disorders of cognitive function.
Conflict of interest statement
Figures


References
-
- Aizawa H, Hu SC, Bobb K, Balakrishnan K, Ince G, et al. Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science. 2004;303:197–202. - PubMed
-
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. - PubMed
-
- Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. - PubMed
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

