A monoclonal antibody marker for the exclusion-zone filaments of Trypanosoma brucei
- PMID: 18616805
- PMCID: PMC2481259
- DOI: 10.1186/1756-3305-1-21
A monoclonal antibody marker for the exclusion-zone filaments of Trypanosoma brucei
Abstract
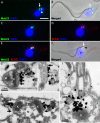
Background: Trypanosoma brucei is a haemoflagellate pathogen of man, wild animals and domesticated livestock in central and southern Africa. In all life cycle stages this parasite has a single mitochondrion that contains a uniquely organised genome that is condensed into a flat disk-like structure called the kinetoplast. The kinetoplast is essential for insect form procyclic cells and therefore is a potential drug target. The kinetoplast is unique in nature because it consists of novel structural proteins and thousands of circular, interlocking, DNA molecules (kDNA). Secondly, kDNA replication is critically timed to coincide with nuclear S phase and new flagellum biogenesis. Thirdly, the kinetoplast is physically attached to the flagellum basal bodies via a structure called the tripartite attachment complex (TAC). The TAC consists of unilateral filaments (within the mitochondrion matrix), differentiated mitochondrial membranes and exclusion-zone filaments that extend from the distal end of the basal bodies. To date only one protein, p166, has been identified to be a component of the TAC.
Results: In the work presented here we provide data based on a novel EM technique developed to label and characterise cytoskeleton structures in permeabilised cells without extraction of mitochondrion membranes. We use this protocol to provide data on a new monoclonal antibody reagent (Mab 22) and illustrate the precise localisation of basal body-mitochondrial linker proteins. Mab 22 binds to these linker proteins (exclusion-zone filaments) and provides a new tool for the characterisation of cytoskeleton mediated kinetoplast segregation.
Conclusion: The antigen(s) recognised by Mab 22 are cytoskeletal, insensitive to extraction by high concentrations of non-ionic detergent, extend from the proximal region of basal bodies and bind to the outer mitochondrial membrane. This protein(s) is the first component of the TAC exclusion-zone fibres to be identified. Mab 22 will therefore be important in characterising TAC biogenesis.
Figures


References
LinkOut - more resources
Full Text Sources

