The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections
- PMID: 18617426
- PMCID: PMC2519120
- DOI: 10.1016/j.immuni.2008.05.011
The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections
Abstract
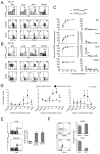
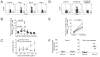
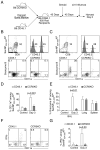
Innate recognition of invading pathogens in peripheral tissues results in the recruitment of circulating memory CD8(+) T cells to sites of localized inflammation during the early phase of a recall response. However, the mechanisms that control the rapid recruitment of these cells to peripheral sites are poorly understood, particularly in relation to influenza and parainfluenza infections of the respiratory tract. In this study, we demonstrate a crucial role for C-C chemokine receptor 5 (CCR5) in the accelerated recruitment of memory CD8(+) T cells to the lung airways during virus challenge. Most importantly, CCR5 deficiency resulted in decreased recruitment of memory T cells expressing key effector molecules and impaired control of virus replication during the initial stages of a secondary response. These data highlight the critical importance of early memory T cell recruitment for the efficacy of cellular immunity in the lung.
Figures




References
-
- Alsharifi M, Lobigs M, Regner M, Lee E, Koskinen A, Mullbacher A. Type I interferons trigger systemic, partial lymphocyte activation in response to viral infection. J Immunol. 2005;175:4635–4640. - PubMed
-
- Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–310. - PubMed
-
- Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–1076. - PubMed
-
- Chvatchko Y, Proudfoot AEI, Buser R, Juillard P, Alouani S, Kosco-Vilbois M, Coyle AJ, Nibbs RJ, Graham G, Offord RE, Wells TNC. Inhibition of Airway Inflammation by Amino-Terminally Modified RANTES/CC Chemokine Ligand 5 Analogues Is Not Mediated through CCR3. J Immunol. 2003;171:5498–5506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

