Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial
- PMID: 18617486
- PMCID: PMC2656923
- DOI: 10.1136/bmj.a257
Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial
Abstract
Objective: To assess the impact of prophylactic oral co-trimoxazole in reducing mortality in HIV positive Zambian adults being treated for pulmonary tuberculosis.
Design: Double blind placebo controlled randomised clinical trial.
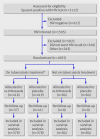
Participants: Two groups of antiretroviral treatment naive adults with HIV infection: patients newly diagnosed as having tuberculosis and receiving tuberculosis treatment either for the first time or for retreatment after relapse; previously treated patients not receiving treatment.
Intervention: Oral co-trimoxazole or matching placebo daily. Primary outcome measures Time to death and occurrence of serious adverse events related to study drug.
Results: 1003 patients were randomised: 835 (416 co-trimoxazole, 419 placebo) were receiving treatment for tuberculosis, 762 (376 co-trimoxazole, 386 placebo) of them newly diagnosed previously untreated patients and 73 (40 co-trimoxazole, 33 placebo) receiving a retreatment regimen; 168 (84 co-trimoxazole, 84 placebo) were not on treatment but had received treatment in the past. Of 835 participants receiving tuberculosis treatment, follow-up information was available for 757, with a total of 1012.6 person years of follow-up. A total of 310 (147 co-trimoxazole, 163 placebo) participants died, corresponding to death rates of 27.3 and 34.4 per 100 person years. In the Cox regression analysis, the hazard ratio for death (co-trimoxazole:placebo) was 0.79 (95% confidence interval 0.63 to 0.99). The effect of co-trimoxazole waned with time, possibly owing to falling adherence levels; in a per protocol analysis based on patients who spent at least 90% of their time at risk supplied with study drug, the hazard ratio was 0.65 (0.45 to 0.93).
Conclusions: Prophylaxis with co-trimoxazole reduces mortality in HIV infected adults with pulmonary tuberculosis. Co-trimoxazole was generally safe and well tolerated.
Trial registration: Current Controlled Trials ISRCTN15281875.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
Prophylaxis with co-trimoxazole for HIV infected adults in Africa.BMJ. 2008 Jul 9;337:a304. doi: 10.1136/bmj.a304. BMJ. 2008. PMID: 18614521 No abstract available.
References
-
- World Health Organization. Global tuberculosis control—surveillance, planning, financing: WHO report 2008. www.who.int/tb/publications/global_report/2008/en/index.html.
-
- Martinson NA, Karstaedt A, Venter WD, Omar T, King P, Mbengo T, et al. Causes of death in hospitalised adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS 2007:21:2043-50. - PubMed
-
- Nyamande K, Lalloo UG, John M. TB presenting as community-acquired pneumonia in a setting of high TB incidence and high HIV prevalence. Int J Tuberc Lung Dis 2007;12:1308-13. - PubMed
-
- Wiktor SZ, Sassan-Morokro M, Grant AD, Abouya L, Karon JM, Maurice C, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Côte d’Ivoire: a randomised controlled trial. Lancet 1999;353:1469-75. - PubMed
-
- Anglaret X, Chêne G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d’Ivoire: a randomised trial. Lancet 1999;353:1463-8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous