Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/l by transient transfection under serum-free conditions
- PMID: 18617574
- PMCID: PMC2528171
- DOI: 10.1093/nar/gkn423
Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/l by transient transfection under serum-free conditions
Abstract
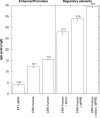
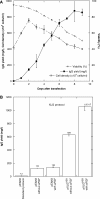
Transient transfection allows for fast production of recombinant proteins. However, the current bottlenecks in transient transfection are low titers and low specific productivity compared to stable cell lines. Here, we report an improved transient transfection protocol that yields titers exceeding 1 g/l in HEK293E cells. This was achieved by combining a new highly efficient polyethyleneimine (PEI)-based transfection protocol, optimized gene expression vectors, use of cell cycle regulators p18 and p21, acidic Fibroblast Growth Factor, exposure of cells to valproic acid and consequently the maintenance of cells at high cell densities (4 million cells/ml). This protocol was reproducibly scaled-up to a working volume of 2 l, thus delivering >1 g of purified protein just 2 weeks after transfection. This is the fastest approach to gram quantities of protein ever reported from cultivated mammalian cells and could initiate, upon further scale-up, a paradigm shift in industrial production of such proteins for any application in biotechnology.
Figures

References
-
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004;22:1393–1398. - PubMed
-
- Baldi L, Hacker DL, Adam M, Wurm FM. Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives. Biotechnol. Lett. 2007;29:677–684. - PubMed
-
- Wurm F, Bernard A. Large-scale transient expression in mammalian cells for recombinant protein production. Curr. Opin. Biotechnol. 1999;10:156–159. - PubMed
-
- Geisse S, Henke M. Large-scale transient transfection of mammalian cells: a newly emerging attractive option for recombinant protein production. J. Struct. Funct. Genomics. 2005;6:165–170. - PubMed
-
- Geisse S, Jordan M, Wurm FM. Large-scale transient expression of therapeutic proteins in mammalian cells. Methods Mol. Biol. 2005;308:87–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

