Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux
- PMID: 18617632
- PMCID: PMC2728020
- DOI: 10.1124/mol.108.048447
Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux
Abstract
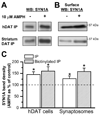
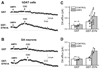
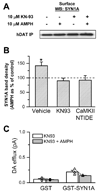
The soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein syntaxin 1A (SYN1A) interacts with and regulates the function of transmembrane proteins, including ion channels and neurotransmitter transporters. Here, we define the first 33 amino acids of the N terminus of the dopamine (DA) transporter (DAT) as the site of direct interaction with SYN1A. Amphetamine (AMPH) increases the association of SYN1A with human DAT (hDAT) in a heterologous expression system (hDAT cells) and with native DAT in murine striatal synaptosomes. Immunoprecipitation of DAT from the biotinylated fraction shows that the AMPH-induced increase in DAT/SYN1A association occurs at the plasma membrane. In a superfusion assay of DA efflux, cells overexpressing SYN1A exhibited significantly greater AMPH-induced DA release with respect to control cells. By combining the patch-clamp technique with amperometry, we measured DA release under voltage clamp. At -60 mV, a physiological resting potential, AMPH did not induce DA efflux in hDAT cells and DA neurons. In contrast, perfusion of exogenous SYN1A (3 microM) into the cell with the whole-cell pipette enabled AMPH-induced DA efflux at -60 mV in both hDAT cells and DA neurons. It has been shown recently that Ca2+/calmodulin-dependent protein kinase II (CaMKII) is activated by AMPH and regulates AMPH-induced DA efflux. Here, we show that AMPH-induced association between DAT and SYN1A requires CaMKII activity and that inhibition of CaMKII blocks the ability of exogenous SYN1A to promote DA efflux. These data suggest that AMPH activation of CaMKII supports DAT/SYN1A association, resulting in a mode of DAT capable of DA efflux.
Figures


References
-
- Arien H, Wiser O, Arkin IT, Leonov H, Atlas D. Syntaxin 1A modulates the voltage-gated L-type calcium channel (Ca(v)1.2) in a cooperative manner. J Biol Chem. 2003;278:29231–29239. - PubMed
-
- Chang SY, Di A, Naren AP, Palfrey HC, Kirk KL, Nelson DJ. Mechanisms of CFTR regulation by syntaxin 1A and PKA. J Cell Sci. 2002;115:783–791. - PubMed
-
- Chapman ER, An S, Barton N, Jahn R. SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem. 1994;269:27427–27432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32-DA020306/DA/NIDA NIH HHS/United States
- DA13975/DA/NIDA NIH HHS/United States
- R01 MH054137/MH/NIMH NIH HHS/United States
- R01 DA013975/DA/NIDA NIH HHS/United States
- DA022413/DA/NIDA NIH HHS/United States
- F31 MH081423/MH/NIMH NIH HHS/United States
- F31 DA021069/DA/NIDA NIH HHS/United States
- K05 DA022413/DA/NIDA NIH HHS/United States
- DA011697/DA/NIDA NIH HHS/United States
- R01 MH063232/MH/NIMH NIH HHS/United States
- P01 DA012408/DA/NIDA NIH HHS/United States
- R01 DA011697/DA/NIDA NIH HHS/United States
- F31-MH081423/MH/NIMH NIH HHS/United States
- T32 MH018870/MH/NIMH NIH HHS/United States
- F31-DA021069/DA/NIDA NIH HHS/United States
- DA012408/DA/NIDA NIH HHS/United States
- F32 DA020306/DA/NIDA NIH HHS/United States
- MH058921/MH/NIMH NIH HHS/United States
- R56 DA013975/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

