Cardiovascular and hematopoietic defects associated with Notch1 activation in embryonic Tie2-expressing populations
- PMID: 18617694
- PMCID: PMC2654335
- DOI: 10.1161/CIRCRESAHA.108.177808
Cardiovascular and hematopoietic defects associated with Notch1 activation in embryonic Tie2-expressing populations
Abstract
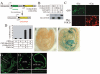
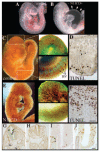
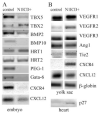
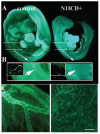
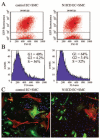
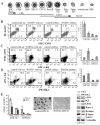
Notch signaling is critical for the development and maintenance of the cardiovasculature, with loss-of-function studies defining roles of Notch1 in the endothelial/hematopoietic lineages. No in vivo studies have addressed complementary gain-of-function strategies within these tissues to define consequences of Notch activation. We developed a transgenic model of Cre recombinase-mediated activation of a constitutively active mouse Notch1 allele (N1ICD(+)) and studied transgene activation in Tie2-expressing lineages. The in vivo phenotype was compared to effects of Notch1 activation on endothelial tubulogenesis, paracrine regulation of smooth muscle cell proliferation, and hematopoiesis. N1ICD(+) embryos showed midgestation lethality with defects in angiogenic remodeling of embryonic and yolk sac vasculature, cardiac development, smooth muscle cell investment of vessels, and hematopoietic differentiation. Angiogenic defects corresponded with impaired endothelial tubulogenesis in vitro following Notch1 activation and paracrine inhibition of smooth muscle cells when grown with Notch1-activated endothelial cells. Flow cytometric analysis of hematopoietic and endothelial precursor populations demonstrated a significant loss of CD71(+)/Ter119(+) populations with an active N1ICD(+) allele and a corresponding increase in c-Kit(+)/CD71 and Flk1(+) populations, suggesting a developmental block during the transition between c-Kit- and Ter119-expressing erythroblasts. Cardiovascular lineages are sensitive to an imbalance in Notch signaling, with aberrant activation reflecting a vascular phenotype comparable to a loss-of-function Notch1 mutation.
Figures


References
-
- Robb L, Elefanty AG. The hemangioblast—an elusive cell captured in culture. Bioessays. 1998;20:611–614. - PubMed
-
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. - PubMed
-
- de Angelis M Hrabe, McIntyre J, II, Gossler A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature. 1997;386:717–721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

