The type VI secretion toolkit
- PMID: 18617888
- PMCID: PMC2515208
- DOI: 10.1038/embor.2008.131
The type VI secretion toolkit
Abstract
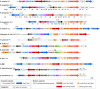
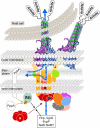
Bacterial secretion systems are macromolecular complexes that release virulence factors into the medium or translocate them into the target host cell. These systems are widespread in bacteria allowing them to infect eukaryotic cells and survive or replicate within them. A new secretion system, the type VI secretion system (T6SS), was recently described and characterized in several pathogens. Genomic data suggest that T6SS exist in most bacteria that come into close contact with eukaryotic cells, including plant and animal pathogens. Many research groups are now investigating the underlying mechanisms and the way in which the effector proteins translocated through this machine subvert host defences. This review provides an overview of our current knowledge about type VI secretion, focusing on gene regulation, components of the secretion machine, substrate secretion and the cellular consequences for the host cell.
Figures


References
-
- Akeda Y, Galán J (2005) Chaperone release and unfolding of substrates in type III secretion. Nature 437: 911–915 - PubMed
-
- Barker J, Klose K (2007) Molecular and genetic basis of pathogenesis in F. tularensis. Ann NY Acad Sci 1105: 138–159 - PubMed
-
- Bingle L, Bailey C, Pallen M (2008) Type VI secretion: a beginner's guide. Curr Opin Microbiol 11: 3–8 - PubMed
-
- Bladergroen M, Badelt K, Spaink H (2003) Infection-blocking genes of a symbiotic R. leguminosarum strain that are involved in temperature-dependent protein secretion. Mol Plant Microbe Interact 16: 53–64 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

