Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2
- PMID: 18618018
- PMCID: PMC2447929
- DOI: 10.1172/JCI35808
Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2
Abstract
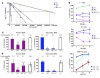
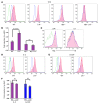
In addition to its role in megakaryocyte production, signaling initiated by thrombopoietin (TPO) activation of its receptor, myeloproliferative leukemia virus protooncogene (c-Mpl, or Mpl), controls HSC homeostasis and self-renewal. Under steady-state conditions, mice lacking the inhibitory adaptor protein Lnk harbor an expanded HSC pool with enhanced self-renewal. We found that HSCs from Lnk-/- mice have an increased quiescent fraction, decelerated cell cycle kinetics, and enhanced resistance to repeat treatments with cytoablative 5-fluorouracil in vivo compared with WT HSCs. We further provide genetic evidence demonstrating that Lnk controls HSC quiescence and self-renewal, predominantly through Mpl. Consistent with this observation, Lnk-/- HSCs displayed potentiated activation of JAK2 specifically in response to TPO. Biochemical experiments revealed that Lnk directly binds to phosphorylated tyrosine residues in JAK2 following TPO stimulation. Of note, the JAK2 V617F mutant, found at high frequencies in myeloproliferative diseases, retains the ability to bind Lnk. Therefore, we identified Lnk as a physiological negative regulator of JAK2 in stem cells and TPO/Mpl/JAK2/Lnk as a major regulatory pathway in controlling stem cell self-renewal and quiescence.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

