Ang II-stimulated migration of vascular smooth muscle cells is dependent on LR11 in mice
- PMID: 18618022
- PMCID: PMC2447934
- DOI: 10.1172/JCI32381
Ang II-stimulated migration of vascular smooth muscle cells is dependent on LR11 in mice
Abstract
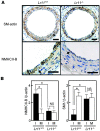
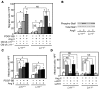
Medial-to-intimal migration of SMCs is critical to atherosclerotic plaque formation and remodeling of injured arteries. Considerable amounts of the shed soluble form of the LDL receptor relative LR11 (sLR11) produced by intimal SMCs enhance SMC migration in vitro via upregulation of urokinase-type plasminogen activator receptor (uPAR) expression. Here, we show that circulating sLR11 is a novel marker of carotid intima-media thickness (IMT) and that targeted disruption of the LR11 gene greatly reduces intimal thickening of arteries through attenuation of Ang II-induced migration of SMCs. Serum concentrations of sLR11 were positively correlated with IMT in dyslipidemic subjects, and multivariable regression analysis suggested sLR11 levels as an index of IMT, independent of classical atherosclerosis risk factors. In Lr11-/- mice, femoral artery intimal thickness after cuff placement was decreased, and Ang II-stimulated migration and attachment of SMCs from these mice were largely abolished. In isolated murine SMCs, sLR11 caused membrane ruffle formation via activation of focal adhesion kinase/ERK/Rac1 accompanied by complex formation between uPAR and integrin alphavbeta3, a process accelerated by Ang II. Overproduction of sLR11 decreased the sensitivity of Ang II-induced activation pathways to inhibition by an Ang II type 1 receptor blocker in mice. Thus, we demonstrate a requirement for sLR11 in Ang II-induced SMC migration and propose what we believe is a novel role for sLR11 as a biomarker of carotid IMT.
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

