Combination of dexamethasone, high-dose cytarabine, and carboplatin is effective for advanced large-cell non-Hodgkin lymphoma of childhood
- PMID: 18618501
- PMCID: PMC2975596
- DOI: 10.1002/cncr.23630
Combination of dexamethasone, high-dose cytarabine, and carboplatin is effective for advanced large-cell non-Hodgkin lymphoma of childhood
Abstract
Background: The purpose of the current study was to evaluate the activity and toxicity of dexamethasone, high-dose cytarabine, and carboplatin (DAC) combination therapy in children with newly diagnosed large-cell non-Hodgkin lymphoma (NHL) and to estimate the event-free and overall survival rates achieved when DAC is incorporated into a conventional regimen.
Methods: From 1991 to 1997, 20 boys and 5 girls aged 4.2 to 17.7 years who had stage III (according to the St. Jude staging system) (n = 21) or stage IV (n = 4) large-cell NHL were treated in this study. DAC therapy was administered at the beginning of the induction phase in 2 sequential cycles and incorporated throughout a continuation phase (modified from the ACOP+ regimen, which features doxorubicin, cyclophosphamide, vincristine, and prednisone) with doxorubicin, cyclophosphamide, vincristine, and dexamethasone. The total duration of treatment was approximately 10 months.
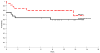
Results: DAC therapy yielded a response in 22 of 25 patients (88%; 95% confidence interval [95% CI], 68%-97%): complete remission in 13 cases (52%), and partial response in 9 (36%). After additional treatment with doxorubicin, cyclophosphamide, vincristine, and dexamethasone, complete remission was attained in 18 patients (72%) and partial remission in 3 (12%). The event-free survival rate (+/- the standard error [SE]) was 64% +/- 9% and the overall survival rate was 80% +/- 8% at 5 years.
Conclusions: The results of the current study indicate that the DAC regimen is well tolerated and effective for pediatric patients with large-cell NHL.
2008 American Cancer Society
Figures
References
-
- National Cancer Institute sponsored study of classifications of non-Hodgkin’s lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin’s Lymphoma Pathologic Classification Project. Cancer. 1982;49:2112–2135. - PubMed
-
- Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001.
-
- Patte C, Philip T, Rodary C, et al. High survival rate in advanced-stage B-cell lymphomas and leukemias without CNS involvement with a short intensive polychemotherapy: results from the French Pediatric Oncology Society of a randomized trial of 216 children. J Clin Oncol. 1991;9:123–132. - PubMed
-
- Schwenn MR, Blattner SR, Lynch E, Weinstein HJ. HiC-COM: a 2-month intensive chemotherapy regimen for children with stage III and IV Burkitt’s lymphoma and B-cell acute lymphoblastic leukemia. J Clin Oncol. 1991;9:133–138. - PubMed
-
- Murphy SB, Bowman WP, Abromowitch M, et al. Results of treatment of advanced-stage Burkitt’s lymphoma and B cell (SIg+) acute lymphoblastic leukemia with high-dose fractionated cyclophosphamide and coordinated high-dose methotrexate and cytarabine. J Clin Oncol. 1986;4:1732–1739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

