Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System
- PMID: 18618511
- PMCID: PMC4188533
- DOI: 10.1002/cncr.23697
Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System
Abstract
Background: Recent studies have highlighted issues with the International Prognostic Scoring System (IPSS) model in relation to the exclusion of many subgroups that now represent a large proportion of patients with myelodysplastic syndrome (MDS) (eg, secondary MDS, chronic myelomonocytic leukemia [CMML] with leukocytosis, prior therapy) and its lack of applicability to most patients on investigational programs, because many would have received prior therapies and would have had MDS for a significant length of time.
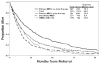
Methods: The authors analyzed 1915 patients with MDS who were referred from 1993 to 2005 (including those with CMML, secondary MDS, and MDS with prior therapy). Only 507 patients (26%) had primary MDS without prior therapy (ie, classifiable by the IPSS). Patients were divided randomly into a study group (n = 958) and a test group (n = 957). RESULTS.: A multivariate analysis of prognostic factors in the study group identified the following adverse, independent factors as continuous and categoric values (P<.001): poor performance, older age, thrombocytopenia, anemia, increased bone marrow blasts, leukocytosis, chromosome 7 or complex (>or=3) abnormalities, and prior transfusions. Cutoffs for anemia, thrombocytopenia and blasts, and cytogenetic subsets were different according to the IPSS. The new MDS prognostic model divided patients into 4 prognostic groups with significantly different outcomes. The model was validated in the test group. Applying the prognostic score of the new model within the 4 IPSS risk groups, overall, and in patients who had primary MDS without prior therapy was found to be highly prognostic in each subset. Applying the IPSS within each of the 4 risk groups of the new MDS model was not found to be prognostic.
Conclusions: The new model accounts for duration of MDS and prior therapy. It is applicable to any patient with MDS at any time during the course of MDS.
(c) 2008 American Cancer Society.
Figures




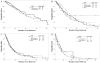


References
-
- Faderl S, Kantarjian H. Myelodysplastic syndromes. In: DeVita V, Hellman S, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. 7. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 2144–2154.
-
- Faderl S, Kantarjian H. Novel therapies for myelodysplastic syndromes. Cancer. 2004;101:226–241. - PubMed
-
- Hoffmann WK, Koeffler HP. Myelodysplastic syndrome. Annu Rev Med. 2005;56:1–16. - PubMed
-
- Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Heamatol. 1982;51:189–199. - PubMed
-
- Harris NJ, Jaffe ES, Diebold J, et al. World Health Organization lymphoid tissues: report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November 1977. J Clin Oncol. 1999;17:3835–3849. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

