Rad51 overexpression rescues radiation resistance in BRCA2-defective cancer cells
- PMID: 18618591
- PMCID: PMC3080251
- DOI: 10.1002/mc.20463
Rad51 overexpression rescues radiation resistance in BRCA2-defective cancer cells
Abstract
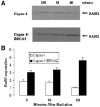
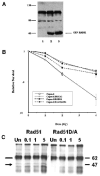
Breast cancers with BRCA2 mutations exhibit DNA repair defects and are particularly sensitive to radiation. BRCA2 interacts with Rad51 in a complex manner involving internal BRC and C-terminal TR2 domains which play a key role in homologous recombination. BRCA2 expression also modulates Rad51 protein levels such that Rad51 protein is relatively decreased in BRCA2-defective cancer cells. This is mediated in part through BRCA2's capacity to protect Rad51 from caspase-3 proteolytic degradation. In order to distinguish between functional and expression related roles for BRCA2 we studied the results of Rad51 overexpression in mouse and human cells with inactivating BRCA2 mutations. The results show that overexpression of wild-type Rad51 partially rescues BRCA2 deficiency but that overexpression of a caspase-3 resistant Rad51 completely complements the BRCA2 defect in radiation responsiveness. These results indicate that Rad51 can compensate for some aspects of a BRCA2 gene defect and suggest that Rad51 expression levels may be an important modifier of the BRCA2 defective genotype.
Copyright 2008 Wiley-Liss, Inc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

