A new in vitro model of the glial scar inhibits axon growth
- PMID: 18618667
- PMCID: PMC3161731
- DOI: 10.1002/glia.20721
A new in vitro model of the glial scar inhibits axon growth
Abstract
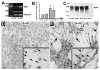
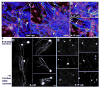
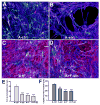
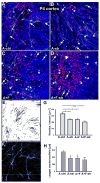
Astrocytes respond to central nervous system (CNS) injury with reactive astrogliosis and participate in the formation of the glial scar, an inhibitory barrier for axonal regeneration. Little is known about the injury-induced mechanisms underlying astrocyte reactivity and subsequent development of an axon-inhibitory scar. We combined two key aspects of CNS injury, mechanical trauma and co-culture with meningeal cells, to produce an in vitro model of the scar from cultures of highly differentiated astrocytes. Our model displayed widespread morphological signs of astrocyte reactivity, increases in expression of glial fibrillary acidic protein (GFAP), and accumulation of GFAP in astrocytic processes. Expression levels of scar-associated markers, phosphacan, neurocan, and tenascins, were also increased. Importantly, neurite growth from various CNS neuronal populations was significantly reduced when neurons were seeded on the scar-like cultures, compared with growth on cultures of mature astrocytes. Quantification of neurite growth parameters on the scar model demonstrated significant reductions in neuronal adhesion and neurite lengths. Interestingly, neurite outgrowth of postnatal neurons was reduced to a greater extent than that of embryonic neurons, and outgrowth inhibition varied among neuronal populations. Scar-like reactive sites and neurite-inhibitory patches were found throughout these cultures, creating a patchwork of growth-inhibitory areas mimicking a CNS injury site. Thus, our model showed relevant aspects of scar formation and produced widespread inhibition of axonal regeneration; it should be useful both for examining mechanisms underlying scar formation and to assess various treatments for their potential to improve regeneration after CNS injury. (c) 2008 Wiley-Liss, Inc.
Figures






References
-
- Abnet K, Fawcett JW, Dunnett SB. Interactions between meningeal cells and astrocytes in vivo and in vitro. Brain Res Dev Brain Res. 1991;59(2):187–96. - PubMed
-
- Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J Neurochem. 2000;74(5):1951–60. - PubMed
-
- Ard MD, Schachner M, Rapp JT, Faissner A. Growth and degeneration of axons on astrocyte surfaces: effects on extracellular matrix and on later axonal growth. Glia. 1993;9(4):248–59. - PubMed
-
- Asher RA, Morgenstern DA, Moon LD, Fawcett JW. Chondroitin sulphate proteoglycans: inhibitory components of the glial scar. Prog Brain Res. 2001;132:611–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

