Synaptic vesicle fusion
- PMID: 18618940
- PMCID: PMC2519048
- DOI: 10.1038/nsmb.1450
Synaptic vesicle fusion
Abstract
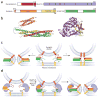
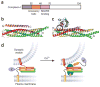
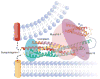
The core of the neurotransmitter release machinery is formed by SNARE complexes, which bring the vesicle and plasma membranes together and are key for fusion, and by Munc18-1, which controls SNARE-complex formation and may also have a direct role in fusion. In addition, SNARE complex assembly is likely orchestrated by Munc13s and RIMs, active-zone proteins that function in vesicle priming and diverse forms of presynaptic plasticity. Synaptotagmin-1 mediates triggering of release by Ca2+, probably through interactions with SNAREs and both membranes, as well as through a tight interplay with complexins. Elucidation of the release mechanism will require a full understanding of the network of interactions among all these proteins and the membranes.
Figures

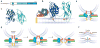
References
-
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. - PubMed
-
- Rosenmund C, Rettig J, Brose N. Molecular mechanisms of active zone function. Curr Opin Neurobiol. 2003;13:509–519. - PubMed
-
- Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q Rev Biophys. 2005;38:1–47. - PubMed
-
- Rizo J, Chen X, Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339–350. - PubMed
-
- Jahn R, Scheller RH. SNAREs — engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

