Progress in matrix metalloproteinase research
- PMID: 18619669
- PMCID: PMC2810947
- DOI: 10.1016/j.mam.2008.05.002
Progress in matrix metalloproteinase research
Abstract
Matrix metalloproteinases (MMPs) are now acknowledged as key players in the regulation of both cell-cell and cell-extracellular matrix interactions. They are involved in modifying matrix structure, growth factor availability and the function of cell surface signalling systems, with consequent effects on cellular differentiation, proliferation and apoptosis. They play central roles in morphogenesis, wound healing, tissue repair and remodelling in response to injury and in the progression of diseases such as arthritis, cancer and cardiovascular disease. Because of their wide spectrum of activities and expression sites, the elucidation of their potential as drug targets in disease or as important features of the repair process will be dependent upon careful analysis of their role in different cellular locations and at different disease stages. Novel approaches to the specific regulation of individual MMPs in different contexts are also being developed.
Figures

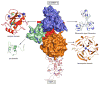
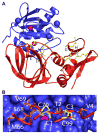
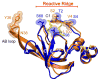
References
-
- Acuff HB, Sinnamon M, Fingleton B, Boone B, Levy SE, Chen X, Pozzi A, Carbone DP, Schwartz DR, Moin K, Sloane BF, Matrisian LM. Analysis of host- and tumor-derived proteinases using a custom dual species microarray reveals a protective role for stromal matrix metalloproteinase-12 in non-small cell lung cancer. Cancer Res. 2006;66 (16):7968–7975. - PubMed
-
- Ahokas K, Lohi J, Illman SA, Llano E, Elomaa O, Impola U, Karjalainen-Lindsberg ML, Saarialho-Kere U. Matrix metalloproteinase-21 is expressed epithelially during development and in cancer and is up-regulated by transforming growth factor-beta1 in keratinocytes. Lab Invest. 2003;83 (12):1887–1899. - PubMed
-
- Ahonen M, Poukkula M, Baker AH, Kashiwagi M, Nagase H, Eriksson JE, Kähäri VM. Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene. 2003;22 (14):2121–2134. - PubMed
-
- Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the speciFIc 3/4- and 1/4-length fragments. J Biol Chem. 1995;270 (11):5872–5876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

