Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors
- PMID: 18621530
- PMCID: PMC2570262
- DOI: 10.1016/j.bmcl.2008.06.052
Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors
Abstract
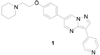
A structure-activity relationship study of dorsomorphin, a previously identified inhibitor of SMAD 1/5/8 phosphorylation by bone morphogenetic protein (BMP) type 1 receptors ALK2, 3, and 6, revealed that increased inhibitory activity could be accomplished by replacing the pendent 4-pyridine ring with 4-quinoline. The activity contributions of various nitrogen atoms in the core pyrazolo[1,5-a]pyrimidine ring were also examined by preparing and evaluating pyrrolo[1,2-a]pyrimidine and pyrazolo[1,5-a]pyridine derivatives. In addition, increased mouse liver microsome stability was achieved by replacing the ether substituent on the pendent phenyl ring with piperazine. Finally, an optimized compound 13 (LDN-193189 or DM-3189) demonstrated moderate pharmacokinetic characteristics (e.g., plasma t(1/2)=1.6h) following intraperitoneal administration in mice. These studies provide useful molecular probes for examining the in vivo pharmacology of BMP signaling inhibition.
Figures




References
-
- Liu D, Wang J, Kinzel B, Müeller M, Mao X, Valdez R, Liu Y, Li E. Blood. 2007;110:1502. - PubMed
-
- Ye L, Lewis-Russell JM, Kyanaston HG, Jiang WG. Histol. Histopathol. 2007;22:1129. - PubMed
-
- Kim IY, Lee DH, Lee DK, Kim BC, Kim HT, Leach FS, Linehan WM, Morton RA, Kim SJ. Clin. Cancer Res. 2003;9:6046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

