Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections
- PMID: 18621682
- PMCID: PMC2474537
- DOI: 10.1073/pnas.0801963105
Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections
Abstract
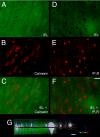
Calcium (Ca(2+)) release through inositol 1,4,5-trisphosphate receptors (IP(3)Rs) regulates the function of virtually every mammalian cell. Unlike ryanodine receptors, which generate local Ca(2+) events ("sparks") that transmit signals to the juxtaposed cell membrane, a similar functional architecture has not been reported for IP(3)Rs. Here, we have identified spatially fixed, local Ca(2+) release events ("pulsars") in vascular endothelial membrane domains that project through the internal elastic lamina to adjacent smooth muscle membranes. Ca(2+) pulsars are mediated by IP(3)Rs in the endothelial endoplasmic reticulum of these membrane projections. Elevation of IP(3) by the endothelium-dependent vasodilator, acetylcholine, increased the frequency of Ca(2+) pulsars, whereas blunting IP(3) production, blocking IP(3)Rs, or depleting endoplasmic reticulum Ca(2+) inhibited these events. The elementary properties of Ca(2+) pulsars were distinct from ryanodine-receptor-mediated Ca(2+) sparks in smooth muscle and from IP(3)-mediated Ca(2+) puffs in Xenopus oocytes. The intermediate conductance, Ca(2+)-sensitive potassium (K(Ca)3.1) channel also colocalized to the endothelial projections, and blockage of this channel caused an 8-mV depolarization. Inhibition of Ca(2+) pulsars also depolarized to a similar extent, and blocking K(Ca)3.1 channels was without effect in the absence of pulsars. Our results support a mechanism of IP(3) signaling in which Ca(2+) release is spatially restricted to transmit intercellular signals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Nelson MT, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. - PubMed
-
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor:Where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. - PubMed
-
- Kohler R, Hoyer J. The endothelium-derived hyperpolarizing factor: Insights from genetic animal models. Kidney Int. 2007;72:145–150. - PubMed
-
- McSherry IN, et al. A role for heterocellular coupling and EETs in dilation of rat cremaster arteries. Microcirculation. 2006;13:119–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK065992/DK/NIDDK NIH HHS/United States
- DK58795/DK/NIDDK NIH HHS/United States
- DK53832/DK/NIDDK NIH HHS/United States
- P01 HL077378/HL/NHLBI NIH HHS/United States
- R37 DK053832/DK/NIDDK NIH HHS/United States
- R01 DK053832/DK/NIDDK NIH HHS/United States
- R01 DK058795/DK/NIDDK NIH HHS/United States
- R01 DK065947/DK/NIDDK NIH HHS/United States
- HL77378/HL/NHLBI NIH HHS/United States
- R01 HL044455/HL/NHLBI NIH HHS/United States
- R01 HL045239/HL/NHLBI NIH HHS/United States
- HL45239/HL/NHLBI NIH HHS/United States
- DK65992/DK/NIDDK NIH HHS/United States
- DK65947/DK/NIDDK NIH HHS/United States
- HL44455/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

