Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors
- PMID: 18621685
- PMCID: PMC2474524
- DOI: 10.1073/pnas.0803644105
Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors
Abstract
Persistent autoantibody production in patients with systemic lupus erythematosus (SLE) suggests the existence of autoreactive humoral memory, but the frequency of self-reactive memory B cells in SLE has not been determined. Here, we report on the reactivity of 200 monoclonal antibodies from single IgG+ memory B cells of four SLE patients. The overall frequency of polyreactive and HEp-2 self-reactive antibodies in this compartment was similar to controls. We found 15% of IgG memory B cell antibodies highly reactive and specific for SLE-associated extractable nuclear antigens (ENA) Ro52 and La in one patient with serum autoantibody titers of the same specificity but not in the other three patients or healthy individuals. The germ-line forms of the ENA antibodies were non-self-reactive or polyreactive with low binding to Ro52, supporting the idea that somatic mutations contributed to autoantibody specificity and reactivity. Heterogeneity in the frequency of memory B cells expressing SLE-associated autoantibodies suggests that this variable may be important in the outcome of therapies that ablate this compartment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

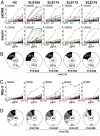
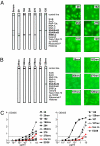
References
-
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. - PubMed
-
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. - PubMed
-
- Ahmed R, Gray D. Immunological memory and protective immunity: Understanding their relation. Science. 1996;272:54–60. - PubMed
-
- Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–350. - PubMed
-
- Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

