Autophagy is an adaptive response in desmin-related cardiomyopathy
- PMID: 18621691
- PMCID: PMC2474535
- DOI: 10.1073/pnas.0706802105
Autophagy is an adaptive response in desmin-related cardiomyopathy
Abstract
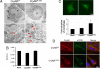
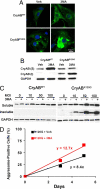
A missense mutation in the alphaB-crystallin (CryAB) gene triggers a severe form of desmin-related cardiomyopathy (DRCM) characterized by accumulation of misfolded proteins. We hypothesized that autophagy increases in response to protein aggregates and that this autophagic activity is adaptive. Mutant CryAB (CryAB(R120G)) triggered a >2-fold increase in cardiomyocyte autophagic activity, and blunting autophagy increased the rate of aggregate accumulation and the abundance of insoluble CryAB(R120G)-associated aggregates. Cardiomyocyte-restricted overexpression of CryAB(R120G) in mice induced intracellular aggregate accumulation and systolic heart failure by 12 months. As early as 2 months (well before the earliest declines in cardiac function), we detected robust autophagic activity. To test the functional significance of autophagic activation, we crossed CryAB(R120G) mice with animals harboring heterozygous inactivation of beclin 1, a gene required for autophagy. Blunting autophagy in vivo dramatically hastened heart failure progression with a 3-fold increase in interstitial fibrosis, greater accumulation of polyubiquitinated proteins, larger and more extensive intracellular aggregates, accelerated ventricular dysfunction, and early mortality. This study reports activation of autophagy in DRCM. Further, our findings point to autophagy as an adaptive response in this proteotoxic form of heart disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. - PubMed
-
- Goebel HH, Warlo IA. Surplus protein myopathies. Neuromuscul Disord. 2001;11:3–6. - PubMed
-
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. - PubMed
-
- Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–2100. - PubMed
-
- Dalakas MC, et al. Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. N Engl J Med. 2000;342:770–780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

