Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis
- PMID: 18621709
- PMCID: PMC2474483
- DOI: 10.1073/pnas.0801527105
Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis
Abstract
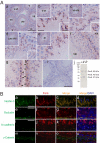
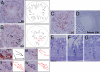
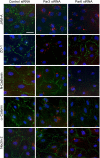
The Par3/Par6/aPKC and the CRB3/Pals1/PATJ polarity complexes are involved in regulating apical ectoplasmic specialization (ES) and blood-testis barrier (BTB) restructuring in the testis. Par6 was a component of the apical ES and the BTB. However, its level was considerably diminished at both sites at stage VIII of the cycle. Par6 also formed a stable complex with Pals1 and JAM-C (a component of the apical ES) in normal testes. When rats were treated with adjudin to induce apical ES restructuring without compromising the BTB, Par6 staining virtually disappeared at the apical ES in misaligned spermatids before their depletion. Additionally, the Par6/Pals1 complex became tightly associated with Src kinase, rendering a loss of association of the Par6/Pals1 complex with JAM-C, thereby destabilizing apical ES to facilitate spermatid loss. Primary Sertoli cell cultures with established functional BTB, but without apical ES, were next used to assess the Par6-based complex on BTB dynamics. When either Par6 or Par3 was knocked down by RNAi in Sertoli cell epithelium, a significant loss of the corresponding protein by approximately 60% in cells vs. controls was detected, alongside with a decline in aPKC after Par6, but not Par3, knockdown. This Par3 or Par6 knockdown also led to a transient loss of selected BTB proteins at the cell-cell interface, thereby compromising the BTB integrity. These findings illustrate that the Par6/Par3-based polarity complex likely coordinates the events of apical ES and BTB restructuring that take place concurrently at the opposing ends of adjacent Sertoli cells in the seminiferous epithelium during spermatogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

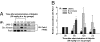

References
-
- Macara IG. Parsing the polarity code. Nat Rev Mol Cell Biol. 2004;5:220–231. - PubMed
-
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. - PubMed
-
- Suzuki A, Ohno S. The Par-aPKC system: Lessons in polarity. J Cell Sci. 2006;119:979–987. - PubMed
-
- Mruk DD, Cheng CY. Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

