The role of nanostructure in the wetting behavior of mixed-monolayer-protected metal nanoparticles
- PMID: 18621710
- PMCID: PMC2453071
- DOI: 10.1073/pnas.0803929105
The role of nanostructure in the wetting behavior of mixed-monolayer-protected metal nanoparticles
Abstract
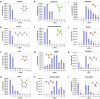
Self-assembled monolayer-protected nanoparticles are promising candidates for applications, such as sensing and drug delivery, in which the molecular ligands' interactions with the surrounding environment play a crucial role. We recently showed that, when gold nanoparticles are coated with a binary mixture of immiscible ligands, ordered ribbon-like domains of alternating composition spontaneously form and that their width is comparable with the size of a single solvent molecule. It is usually assumed that nanoparticles' solubility depends solely on the core size and on the molecular composition of the ligand shell. Here, we show that this is not always the case. We find that the ligand shell morphology affects the solubility of these nanoparticles almost as much as the molecular composition. A possible explanation is offered through a molecular dynamics analysis of the surface energy of monolayers differing only in their domain structure. We find that the surface free energy of such model systems can vary significantly as a function of ordering, even at fixed composition. This combined experimental and theoretical study provides a unique insight into wetting phenomena at the nano- and subnanometer scale.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

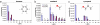

References
-
- Templeton AC, Wuelfing MP, Murray RW. Monolayer protected cluster molecules. Acc Chem Res. 2000;33:27–36. - PubMed
-
- Daniel MC, Astruc D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. - PubMed
-
- Thomas KG, Kamat PV. Chromophore-functionalized gold nanoparticles. Acc Chem Res. 2003;36:888–898. - PubMed
-
- Shipway AN, Katz E, Willner I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. ChemPhysChem. 2000;1:18–52. - PubMed
-
- Jimenez VL, Leopold MC, Mazzitelli C, Jorgenson JW, Murray RW. HPLC of monolayer-protected gold nanoclusters. Anal Chem. 2003;75:199–206. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

