c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation
- PMID: 18621737
- PMCID: PMC3259840
- DOI: 10.1074/jbc.C800128200
c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation
Abstract
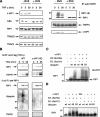
The inhibitor of apoptosis (IAP) proteins are a family of anti-apoptotic regulators found in viruses and metazoans. c-IAP1 and c-IAP2 are recruited to tumor necrosis factor receptor 1 (TNFR1)-associated complexes where they can regulate receptor-mediated signaling. Both c-IAP1 and c-IAP2 have been implicated in TNFalpha-stimulated NF-kappaB activation. However, individual c-IAP1 and c-IAP2 gene knock-outs in mice did not reveal changes in TNF signaling pathways, and the phenotype of a combined deficiency of c-IAPs has yet to be reported. Here we investigate the role of c-IAP1 and c-IAP2 in TNFalpha-stimulated activation of NF-kappaB. We demonstrate that TNFalpha-induced NF-kappaB activation is severely diminished in the absence of both c-IAP proteins. In addition, combined absence of c-IAP1 and c-IAP2 rendered cells sensitive to TNFalpha-induced cell death. Using cells with genetic ablation of c-IAP1 or cells where the c-IAP proteins were eliminated using IAP antagonists, we show that TNFalpha-induced RIP1 ubiquitination is abrogated in the absence of c-IAPs. Furthermore, we reconstitute the ubiquitination process with purified components in vitro and demonstrate that c-IAP1, in collaboration with the ubiquitin conjugating enzyme (E2) enzyme UbcH5a, mediates polymerization of Lys-63-linked chains on RIP1. Therefore, c-IAP1 and c-IAP2 are required for TNFalpha-stimulated RIP1 ubiquitination and NF-kappaB activation.
Figures

Similar articles
-
Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination.Cell Death Dis. 2015 Jun 25;6(6):e1800. doi: 10.1038/cddis.2015.158. Cell Death Dis. 2015. PMID: 26111062 Free PMC article.
-
Ubiquitin binding modulates IAP antagonist-stimulated proteasomal degradation of c-IAP1 and c-IAP2(1).Biochem J. 2009 Jan 1;417(1):149-60. doi: 10.1042/BJ20081885. Biochem J. 2009. PMID: 18939944
-
c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling.EMBO J. 2010 Dec 15;29(24):4198-209. doi: 10.1038/emboj.2010.300. Epub 2010 Nov 26. EMBO J. 2010. PMID: 21113135 Free PMC article.
-
(Un)expected roles of c-IAPs in apoptotic and NFkappaB signaling pathways.Cell Cycle. 2008 Jun 1;7(11):1511-21. doi: 10.4161/cc.7.11.5959. Epub 2008 Mar 16. Cell Cycle. 2008. PMID: 18469528 Review.
-
IAP family of cell death and signaling regulators.Methods Enzymol. 2014;545:35-65. doi: 10.1016/B978-0-12-801430-1.00002-0. Methods Enzymol. 2014. PMID: 25065885 Review.
Cited by
-
Requirement of nuclear factor κB for Smac mimetic-mediated sensitization of pancreatic carcinoma cells for gemcitabine-induced apoptosis.Neoplasia. 2011 Dec;13(12):1162-70. doi: 10.1593/neo.11460. Neoplasia. 2011. PMID: 22241962 Free PMC article.
-
Intermediate domain of receptor-interacting protein kinase 1 (RIPK1) determines switch between necroptosis and RIPK1 kinase-dependent apoptosis.J Biol Chem. 2012 Apr 27;287(18):14863-72. doi: 10.1074/jbc.M111.288670. Epub 2012 Feb 23. J Biol Chem. 2012. PMID: 22362767 Free PMC article.
-
Targeting IAP proteins for therapeutic intervention in cancer.Nat Rev Drug Discov. 2012 Feb 1;11(2):109-24. doi: 10.1038/nrd3627. Nat Rev Drug Discov. 2012. PMID: 22293567 Review.
-
Smac mimetic induces cell death in a large proportion of primary acute myeloid leukemia samples, which correlates with defined molecular markers.Oncotarget. 2016 Aug 2;7(31):49539-49551. doi: 10.18632/oncotarget.10390. Oncotarget. 2016. PMID: 27385100 Free PMC article.
-
Emerging Roles for Noncanonical NF-κB Signaling in the Modulation of Inflammatory Bowel Disease Pathobiology.Inflamm Bowel Dis. 2016 Sep;22(9):2265-79. doi: 10.1097/MIB.0000000000000858. Inflamm Bowel Dis. 2016. PMID: 27508514 Free PMC article. Review.
References
-
- Rothe, M., Pan, M. G., Henzel, W. J., Ayres, T. M., and Goeddel, D. V. (1995) Cell 83 1243–1252 - PubMed
-
- Wang, C. Y., Mayo, M. W., Korneluk, R. G., Goeddel, D. V., and Baldwin, A. S., Jr. (1998) Science 281 1680–1683 - PubMed
-
- Samuel, T., Welsh, K., Lober, T., Togo, S. H., Zapata, J. M., and Reed, J. C. (2006) J. Biol. Chem. 281 1080–1090 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

