Design of a disulfide-less, pharmacologically inert, and chemically competent analog of maurocalcine for the efficient transport of impermeant compounds into cells
- PMID: 18621738
- PMCID: PMC2652642
- DOI: 10.1074/jbc.M804727200
Design of a disulfide-less, pharmacologically inert, and chemically competent analog of maurocalcine for the efficient transport of impermeant compounds into cells
Abstract
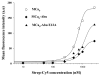
Maurocalcine is a 33-mer peptide initially isolated from the venom of a Tunisian scorpion. It has proved itself valuable as a pharmacological activator of the ryanodine receptor and has helped the understanding of the molecular basis underlying excitation-contraction coupling in skeletal muscles. Because of its positively charged nature, it is also an innovative vector for the cell penetration of various compounds. We report a novel maurocalcine analog with improved properties: (i) the complete loss of pharmacological activity, (ii) preservation of the potent ability to carry cargo molecules into cells, and (iii) coupling chemistries not affected by the presence of internal cysteine residues of maurocalcine. We did this by replacing the six internal cysteine residues of maurocalcine by isosteric 2-aminobutyric acid residues and by adding an additional N-terminal biotinylated lysine (for a proof of concept analog) or an N-terminal cysteine residue (for a chemically competent coupling analogue). Additional replacement of a glutamate residue by alanyl at position 12 further improves the potency of these analogues. Coupling to several cargo molecules or nanoparticles are presented to illustrate the cell penetration potency and usefulness of these pharmacologically inactive analogs.
Figures

Similar articles
-
Small efficient cell-penetrating peptides derived from scorpion toxin maurocalcine.J Biol Chem. 2012 May 18;287(21):17331-17342. doi: 10.1074/jbc.M112.360628. Epub 2012 Mar 20. J Biol Chem. 2012. PMID: 22433862 Free PMC article.
-
Critical amino acid residues of maurocalcine involved in pharmacology, lipid interaction and cell penetration.Biochim Biophys Acta. 2007 Oct;1768(10):2528-40. doi: 10.1016/j.bbamem.2007.06.030. Epub 2007 Jul 10. Biochim Biophys Acta. 2007. PMID: 17888395
-
D-Maurocalcine, a pharmacologically inert efficient cell-penetrating peptide analogue.J Biol Chem. 2010 Oct 29;285(44):34168-80. doi: 10.1074/jbc.M110.104919. Epub 2010 Jul 7. J Biol Chem. 2010. PMID: 20610396 Free PMC article.
-
Maurocalcine and domain A of the II-III loop of the dihydropyridine receptor Cav 1.1 subunit share common binding sites on the skeletal ryanodine receptor.J Biol Chem. 2005 Feb 11;280(6):4013-6. doi: 10.1074/jbc.C400433200. Epub 2004 Dec 9. J Biol Chem. 2005. PMID: 15591063 Free PMC article.
-
Scorpion venom peptides with no disulfide bridges: a review.Peptides. 2014 Jan;51:35-45. doi: 10.1016/j.peptides.2013.10.021. Epub 2013 Oct 31. Peptides. 2014. PMID: 24184590 Review.
Cited by
-
Exploring the Chemical Features and Biomedical Relevance of Cell-Penetrating Peptides.Int J Mol Sci. 2024 Dec 25;26(1):59. doi: 10.3390/ijms26010059. Int J Mol Sci. 2024. PMID: 39795918 Free PMC article. Review.
-
Staurosporine tethered peptide ligands that target cAMP-dependent protein kinase (PKA): optimization and selectivity profiling.Bioorg Med Chem. 2009 Sep 1;17(17):6196-202. doi: 10.1016/j.bmc.2009.07.056. Epub 2009 Jul 26. Bioorg Med Chem. 2009. PMID: 19674907 Free PMC article.
-
Multiple actions of phi-LITX-Lw1a on ryanodine receptors reveal a functional link between scorpion DDH and ICK toxins.Proc Natl Acad Sci U S A. 2013 May 28;110(22):8906-11. doi: 10.1073/pnas.1214062110. Epub 2013 May 13. Proc Natl Acad Sci U S A. 2013. PMID: 23671114 Free PMC article.
-
Biodistribution, Stability, and Blood Distribution of the Cell Penetrating Peptide Maurocalcine in Mice.Int J Mol Sci. 2015 Nov 19;16(11):27730-40. doi: 10.3390/ijms161126054. Int J Mol Sci. 2015. PMID: 26610471 Free PMC article.
-
Imperatoxin A, a Cell-Penetrating Peptide from Scorpion Venom, as a Probe of Ca-Release Channels/Ryanodine Receptors.Pharmaceuticals (Basel). 2010 Apr 1;3(4):1093-1107. doi: 10.3390/ph3041093. Pharmaceuticals (Basel). 2010. PMID: 20668646 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

