Conformational changes in the selectivity filter of the open-state KcsA channel: an energy minimization study
- PMID: 18621821
- PMCID: PMC2547464
- DOI: 10.1529/biophysj.108.136556
Conformational changes in the selectivity filter of the open-state KcsA channel: an energy minimization study
Abstract
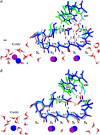
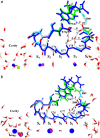
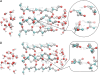
Potassium channels switch between closed and open conformations and selectively conduct K(+) ions. There are at least two gates. The TM2 bundle at the intracellular site is the primary gate of KcsA, and rearrangements at the selectivity filter (SF) act as the second gate. The SF blocks ion flow via an inactivation process similar to C-type inactivation of voltage-gated K(+) channels. We recently generated the open-state conformation of the KcsA channel. We found no major, possibly inactivating, structural changes in the SF associated with this massive inner-pore rearrangement, which suggests that the gates might act independently. Here we energy-minimize the open state of wild-type and mutant KcsA, validating in silico structures of energy-minimized SFs by comparison with crystallographic structures, and use these data to gain insight into how mutation, ion depletion, and K(+) to Na(+) substitution influence SF conformation. Both E71 or D80 protonations/mutations and the presence/absence of protein-buried water molecule(s) modify the H-bonding network stabilizing the P-loops, spawning numerous SF conformations. We find that the inactivated state corresponds to conformations with a partially unoccupied or an entirely empty SF. These structures, involving modifications in all four P-loops, are stabilized by H-bonds between amide H and carbonyl O atoms from adjacent P-loops, which block ion passage. The inner portions of the P-loops are more rigid than the outer parts. Changes are localized to the outer binding sites, with innermost site S4 persisting in the inactivated state. Strong binding by Na(+) locally contracts the SF around Na(+), releasing ligands that do not participate in Na(+) coordination, and occluding the permeation pathway. K(+) selectivity primarily appears to arise from the inability of the SF to completely dehydrate Na(+) ions due to basic structural differences between liquid water and the "quasi-liquid" SF matrix.
Figures

Similar articles
-
Mechanism for selectivity-inactivation coupling in KcsA potassium channels.Proc Natl Acad Sci U S A. 2011 Mar 29;108(13):5272-7. doi: 10.1073/pnas.1014186108. Epub 2011 Mar 14. Proc Natl Acad Sci U S A. 2011. PMID: 21402935 Free PMC article.
-
Sodium permeability and sensitivity induced by mutations in the selectivity filter of the KcsA channel towards Kir channels.Biochimie. 2010 Mar;92(3):232-44. doi: 10.1016/j.biochi.2009.11.007. Epub 2009 Dec 3. Biochimie. 2010. PMID: 19962419
-
Protonation state of E71 in KcsA and its role for channel collapse and inactivation.Proc Natl Acad Sci U S A. 2012 Sep 18;109(38):15265-70. doi: 10.1073/pnas.1211900109. Epub 2012 Aug 31. Proc Natl Acad Sci U S A. 2012. PMID: 22942391 Free PMC article.
-
Importance of hydration and dynamics on the selectivity of the KcsA and NaK channels.J Gen Physiol. 2007 Feb;129(2):135-43. doi: 10.1085/jgp.200609633. Epub 2007 Jan 16. J Gen Physiol. 2007. PMID: 17227917 Free PMC article. Review.
-
Diverse gating in K+ channels: differential role of the pore-helix glutamate in stabilizing the channel pore.Biochem Biophys Res Commun. 2011 Sep 16;413(1):1-4. doi: 10.1016/j.bbrc.2011.08.062. Epub 2011 Aug 22. Biochem Biophys Res Commun. 2011. PMID: 21872570 Review.
Cited by
-
K+/Na+ selectivity in toy cation binding site models is determined by the 'host'.Biophys J. 2009 May 20;96(10):3887-96. doi: 10.1016/j.bpj.2008.12.3963. Biophys J. 2009. PMID: 19450462 Free PMC article.
-
A simple recipe for setting up the flux equations of cyclic and linear reaction schemes of ion transport with a high number of states: The arrow scheme.Channels (Austin). 2016;10(2):119-38. doi: 10.1080/19336950.2015.1120391. Epub 2015 Dec 8. Channels (Austin). 2016. PMID: 26646356 Free PMC article.
-
A molecular mechanism for proton-dependent gating in KcsA.FEBS Lett. 2010 Mar 19;584(6):1126-32. doi: 10.1016/j.febslet.2010.02.003. Epub 2010 Feb 6. FEBS Lett. 2010. PMID: 20138880 Free PMC article.
-
Using a five-state model for fitting amplitude histograms from MaxiK channels: beta-distributions reveal more than expected.Eur Biophys J. 2009 Oct;38(8):1101-14. doi: 10.1007/s00249-009-0515-0. Epub 2009 Jul 21. Eur Biophys J. 2009. PMID: 19626320
-
Discovery and characterisation of a novel toxin from Dendroaspis angusticeps, named Tx7335, that activates the potassium channel KcsA.Sci Rep. 2016 Apr 5;6:23904. doi: 10.1038/srep23904. Sci Rep. 2016. PMID: 27044983 Free PMC article.
References
-
- Doyle, D. A., J. M. Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. - PubMed
-
- Boiteux, C., S. Kraszewski, C. Ramseyer, and C. Girardet. 2007. Ion conductance vs. pore gating and selectivity in KcsA channel: modeling achievements and perspectives. J. Mol. Model. 13:699–713. - PubMed
-
- Berneche, S., and B. Roux. 2005. A gate in the selectivity filter of potassium channels. Structure. 13:591–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

