Charged surface area of maurocalcine determines its interaction with the skeletal ryanodine receptor
- PMID: 18621823
- PMCID: PMC2547443
- DOI: 10.1529/biophysj.107.120840
Charged surface area of maurocalcine determines its interaction with the skeletal ryanodine receptor
Abstract
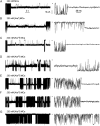
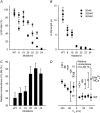
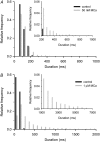
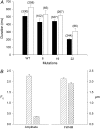
The 33 amino acid scorpion toxin maurocalcine (MCa) has been shown to modify the gating of the skeletal-type ryanodine receptor (RyR1). Here we explored the effects of MCa and its mutants ([Ala(8)]MCa, [Ala(19)]MCa, [Ala(20)]MCa, [Ala(22)]MCa, [Ala(23)]MCa, and [Ala(24)]MCa) on RyR1 incorporated into artificial lipid bilayers and on elementary calcium release events (ECRE) in rat and frog skeletal muscle fibers. The peptides induced long-lasting subconductance states (LLSS) on RyR1 that lasted for several seconds. However, their average length and frequency were decreased if the mutation was placed farther away in the 3D structure from the critical (24)Arg residue. The effect was strongly dependent on the direction of the current through the channel. If the direction was similar to that followed by calcium during release, the peptides were 8- to 10-fold less effective. In fibers long-lasting calcium release events were observed after the addition of the peptides. The average length of these events correlated well with the duration of LLSS. These data suggest that the effect of the peptide is governed by the large charged surface formed by residues Lys(20), Lys(22), Arg(23), Arg(24), and Lys(8). Our observations also indicate that the results from bilayer experiments mimic the in situ effects of MCa on RyR1.
Figures




Similar articles
-
Critical amino acid residues determine the binding affinity and the Ca2+ release efficacy of maurocalcine in skeletal muscle cells.J Biol Chem. 2003 Sep 26;278(39):37822-31. doi: 10.1074/jbc.M305798200. Epub 2003 Jul 17. J Biol Chem. 2003. PMID: 12869557
-
Differential effects of maurocalcine on Ca2+ release events and depolarization-induced Ca2+ release in rat skeletal muscle.J Physiol. 2005 Jun 15;565(Pt 3):843-53. doi: 10.1113/jphysiol.2005.086074. Epub 2005 Apr 14. J Physiol. 2005. PMID: 15831537 Free PMC article.
-
Cell penetration properties of maurocalcine, a natural venom peptide active on the intracellular ryanodine receptor.Biochim Biophys Acta. 2006 Mar;1758(3):308-19. doi: 10.1016/j.bbamem.2006.02.007. Epub 2006 Mar 3. Biochim Biophys Acta. 2006. PMID: 16545341
-
Maurocalcine interacts with the cardiac ryanodine receptor without inducing channel modification.Biochem J. 2007 Sep 1;406(2):309-15. doi: 10.1042/BJ20070453. Biochem J. 2007. PMID: 17537000 Free PMC article.
-
Peptide and protein modulation of local Ca2+ release events in permeabilized skeletal muscle fibers.Biol Res. 2004;37(4):613-6. doi: 10.4067/s0716-97602004000400016. Biol Res. 2004. PMID: 15709689 Review.
Cited by
-
Toxin bioportides: exploring toxin biological activity and multifunctionality.Cell Mol Life Sci. 2017 Feb;74(4):647-661. doi: 10.1007/s00018-016-2343-6. Epub 2016 Aug 23. Cell Mol Life Sci. 2017. PMID: 27554773 Free PMC article. Review.
-
Indian Ornamental Tarantula (Poecilotheria regalis) Venom Affects Myoblast Function and Causes Skeletal Muscle Damage.Cells. 2023 Aug 15;12(16):2074. doi: 10.3390/cells12162074. Cells. 2023. PMID: 37626884 Free PMC article.
-
Scorpion peptides: potential use for new drug development.J Toxicol. 2013;2013:958797. doi: 10.1155/2013/958797. Epub 2013 Jun 15. J Toxicol. 2013. PMID: 23843786 Free PMC article.
-
Structure-function relationships of peptides forming the calcin family of ryanodine receptor ligands.J Gen Physiol. 2016 May;147(5):375-94. doi: 10.1085/jgp.201511499. J Gen Physiol. 2016. PMID: 27114612 Free PMC article.
-
Small efficient cell-penetrating peptides derived from scorpion toxin maurocalcine.J Biol Chem. 2012 May 18;287(21):17331-17342. doi: 10.1074/jbc.M112.360628. Epub 2012 Mar 20. J Biol Chem. 2012. PMID: 22433862 Free PMC article.
References
-
- Tsugorka, A., E. Rios, and L. A. Blatter. 1995. Imaging elementary events of calcium release in skeletal muscle cells. Science. 269:1723–1726. - PubMed
-
- Klein, M. G., H. Cheng, L. F. Santana, Y. H. Jang, W. J. Lederer, and M. F. Schneider. 1996. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 379:455–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

