Conditioning the heart induces formation of signalosomes that interact with mitochondria to open mitoKATP channels
- PMID: 18621853
- PMCID: PMC2544503
- DOI: 10.1152/ajpheart.00520.2008
Conditioning the heart induces formation of signalosomes that interact with mitochondria to open mitoKATP channels
Abstract
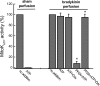
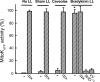
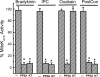
Perfusion of the heart with bradykinin triggers cellular signaling events that ultimately cause opening of mitochondrial ATP-sensitive K+ (mitoKATP) channels, increased H2O2 production, inhibition of the mitochondrial permeability transition (MPT), and cardioprotection. We hypothesized that the interaction of bradykinin with its receptor induces the assembly of a caveolar signaling platform (signalosome) that contains the enzymes of the signaling pathway and that migrates to mitochondria to induce mitoKATP channel opening. We developed a novel method for isolating and purifying signalosomes from Langendorff-perfused rat hearts treated with bradykinin. Fractions containing the signalosomes were found to open mitoKATP channels in mitochondria isolated from untreated hearts via the activation of mitochondrial PKC-epsilon. mitoKATP channel opening required signalosome-dependent phosphorylation of an outer membrane protein. Immunodetection analysis revealed the presence of the bradykinin B2 receptor only in the fraction isolated from bradykinin-treated hearts. Immunodetection and immunogold labeling of caveolin-3, as well as sensitivity to cholesterol depletion and resistance to Triton X-100, attested to the caveolar nature of the signalosomes. Ischemic preconditioning, ischemic postconditioning, and perfusion with ouabain also led to active signalosome fractions that opened mitoKATP channels in mitochondria from untreated hearts. These results provide initial support for a novel mechanism for signal transmission from a plasma membrane receptor to mitoKATP channels.
Figures






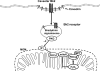
Comment in
-
Signalosomes: delivering cardioprotective signals from GPCRs to mitochondria.Am J Physiol Heart Circ Physiol. 2008 Sep;295(3):H920-H922. doi: 10.1152/ajpheart.00738.2008. Epub 2008 Jul 25. Am J Physiol Heart Circ Physiol. 2008. PMID: 18660439 Free PMC article. No abstract available.
Similar articles
-
Mitochondrial reactive oxygen species: which ROS signals cardioprotection?Am J Physiol Heart Circ Physiol. 2013 Oct 1;305(7):H960-8. doi: 10.1152/ajpheart.00858.2012. Epub 2013 Aug 2. Am J Physiol Heart Circ Physiol. 2013. PMID: 23913710 Free PMC article.
-
Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection.Am J Physiol Heart Circ Physiol. 2004 Jan;286(1):H468-76. doi: 10.1152/ajpheart.00360.2003. Epub 2003 Sep 4. Am J Physiol Heart Circ Physiol. 2004. PMID: 12958031
-
Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT.Am J Physiol Heart Circ Physiol. 2008 Aug;295(2):H874-82. doi: 10.1152/ajpheart.01189.2007. Epub 2008 Jun 27. Am J Physiol Heart Circ Physiol. 2008. PMID: 18586884 Free PMC article.
-
Cardioprotective signaling to mitochondria.J Mol Cell Cardiol. 2009 Jun;46(6):858-66. doi: 10.1016/j.yjmcc.2008.11.019. Epub 2008 Dec 11. J Mol Cell Cardiol. 2009. PMID: 19118560 Free PMC article. Review.
-
cGMP signalling in pre- and post-conditioning: the role of mitochondria.Cardiovasc Res. 2008 Jan 15;77(2):344-52. doi: 10.1093/cvr/cvm050. Epub 2007 Oct 25. Cardiovasc Res. 2008. PMID: 18006449 Review.
Cited by
-
Mitochondria-localized caveolin in adaptation to cellular stress and injury.FASEB J. 2012 Nov;26(11):4637-49. doi: 10.1096/fj.12-215798. Epub 2012 Aug 2. FASEB J. 2012. PMID: 22859372 Free PMC article.
-
Disruption of caveolae blocks ischemic preconditioning-mediated S-nitrosylation of mitochondrial proteins.Antioxid Redox Signal. 2012 Jan 1;16(1):45-56. doi: 10.1089/ars.2010.3844. Epub 2011 Aug 11. Antioxid Redox Signal. 2012. PMID: 21834687 Free PMC article.
-
A refined analysis of superoxide production by mitochondrial sn-glycerol 3-phosphate dehydrogenase.J Biol Chem. 2012 Dec 14;287(51):42921-35. doi: 10.1074/jbc.M112.397828. Epub 2012 Nov 2. J Biol Chem. 2012. PMID: 23124204 Free PMC article.
-
Mitochondrial reactive oxygen species: which ROS signals cardioprotection?Am J Physiol Heart Circ Physiol. 2013 Oct 1;305(7):H960-8. doi: 10.1152/ajpheart.00858.2012. Epub 2013 Aug 2. Am J Physiol Heart Circ Physiol. 2013. PMID: 23913710 Free PMC article.
-
Impact of hypoglycemic agents on myocardial ischemic preconditioning.World J Diabetes. 2014 Jun 15;5(3):258-66. doi: 10.4239/wjd.v5.i3.258. World J Diabetes. 2014. PMID: 24936247 Free PMC article. Review.
References
-
- Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol 291: H2067–H2074, 2006. - PubMed
-
- Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCɛ and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCɛ-MAPK interactions and differential MAPK activation in PKCɛ-induced cardioprotection. Circ Res 90: 390–397, 2002. - PubMed
-
- Balbis A, Baquiran G, Dumas V, Posner BI. Effect of inhibiting vacuolar acidification on insulin signaling in hepatocytes. J Biol Chem 279: 12777–12785, 2004. - PubMed
-
- Bauman AL, Michel JJ, Henson E, Dodge-Kafka KL, Kapiloff MS. The mAKAP signalosome and cardiac myocyte hypertrophy. IUBMB Life 59: 163–169, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

