Distinct contributions of the nisin biosynthesis enzymes NisB and NisC and transporter NisT to prenisin production by Lactococcus lactis
- PMID: 18621866
- PMCID: PMC2546625
- DOI: 10.1128/AEM.00342-08
Distinct contributions of the nisin biosynthesis enzymes NisB and NisC and transporter NisT to prenisin production by Lactococcus lactis
Abstract
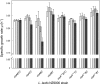
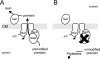
Several Lactococcus lactis strains produce the lantibiotic nisin. The dedicated enzymes NisB and NisC and the transporter NisT modify and secrete the ribosomally synthesized nisin precursor peptide. NisB can function in the absence of the cyclase NisC, yielding the dehydrated prenisin that lacks the thioether rings. A kinetic analysis of nisin production by L. lactis NZ9700 demonstrated that the prenisin was released from the cell into the medium before the processing of the leader sequence occurred. Upon the deletion of nisC, the production of prenisin was reduced by 70%, while in the absence of nisB, the production of prenisin was nearly completely abolished. In cells lacking nisT, no secretion was observed, while the expression of nisABC in these cells resulted in considerable growth rate inhibition caused by the intracellular accumulation of active nisin. Overall, these data indicate that the efficiency of prenisin transport by NisT is markedly enhanced by NisB, suggesting a channeling mechanism of prenisin transfer between the nisin modification enzymes and the transporter.
Figures
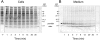


Similar articles
-
Isolation and Analysis of the Nisin Biosynthesis Complex NisBTC: further Insights into Their Cooperative Action.mBio. 2021 Oct 26;12(5):e0258521. doi: 10.1128/mBio.02585-21. Epub 2021 Oct 5. mBio. 2021. PMID: 34607454 Free PMC article.
-
Determining sites of interaction between prenisin and its modification enzymes NisB and NisC.Mol Microbiol. 2011 Nov;82(3):706-18. doi: 10.1111/j.1365-2958.2011.07846.x. Mol Microbiol. 2011. PMID: 22011325
-
Subcellular Localization and Assembly Process of the Nisin Biosynthesis Machinery in Lactococcus lactis.mBio. 2020 Nov 10;11(6):e02825-20. doi: 10.1128/mBio.02825-20. mBio. 2020. PMID: 33173006 Free PMC article.
-
Genetic determinants for the biosynthesis of nisin, a bacteriocin produced by Lactococcus lactis.Microbiologia. 1996 Mar;12(1):61-74. Microbiologia. 1996. PMID: 9019136 Review.
-
Nisin: From a structural and meat preservation perspective.Food Microbiol. 2023 May;111:104207. doi: 10.1016/j.fm.2022.104207. Epub 2022 Dec 9. Food Microbiol. 2023. PMID: 36681394 Review.
Cited by
-
Mechanistic Understanding of Lanthipeptide Biosynthetic Enzymes.Chem Rev. 2017 Apr 26;117(8):5457-5520. doi: 10.1021/acs.chemrev.6b00591. Epub 2017 Jan 30. Chem Rev. 2017. PMID: 28135077 Free PMC article. Review.
-
Substrate recognition and specificity of the NisB protein, the lantibiotic dehydratase involved in nisin biosynthesis.J Biol Chem. 2011 Sep 2;286(35):30552-30560. doi: 10.1074/jbc.M111.263210. Epub 2011 Jul 8. J Biol Chem. 2011. PMID: 21757717 Free PMC article.
-
Genomic and Metabolic Characterization of Plant Growth-Promoting Rhizobacteria Isolated from Nodules of Clovers Grown in Non-Farmed Soil.Int J Mol Sci. 2023 Nov 23;24(23):16679. doi: 10.3390/ijms242316679. Int J Mol Sci. 2023. PMID: 38069003 Free PMC article.
-
Isolation and Analysis of the Nisin Biosynthesis Complex NisBTC: further Insights into Their Cooperative Action.mBio. 2021 Oct 26;12(5):e0258521. doi: 10.1128/mBio.02585-21. Epub 2021 Oct 5. mBio. 2021. PMID: 34607454 Free PMC article.
-
Impact of the nisin modification machinery on the transport kinetics of NisT.Sci Rep. 2020 Jul 23;10(1):12295. doi: 10.1038/s41598-020-69225-2. Sci Rep. 2020. PMID: 32703992 Free PMC article.
References
-
- Chatterjee, C., M. Paul, L. Xie, and W. A. van der Donk. 2005. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105:633-684. - PubMed
-
- Gross, E., and J. L. Morell. 1971. The structure of nisin. J. Am. Chem. Soc. 93:4634-4635. - PubMed
-
- Hasper, H. E., N. E. Kramer, J. L. Smith, J. D. Hillman, C. Zachariah, O. P. Kuipers, B. de Kruijff, and E. Breukink. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636-1637. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

