Polo-like kinase is expressed in S/G2/M phase and associated with the flagellum attachment zone in both procyclic and bloodstream forms of Trypanosoma brucei
- PMID: 18621923
- PMCID: PMC2547065
- DOI: 10.1128/EC.00150-08
Polo-like kinase is expressed in S/G2/M phase and associated with the flagellum attachment zone in both procyclic and bloodstream forms of Trypanosoma brucei
Abstract
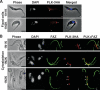
Trypanosoma brucei, the etiologic agent of African sleeping sickness, divides into insect (procyclic) and bloodstream forms. These two forms are subject to distinct cell cycle regulations, with cytokinesis controlled primarily by basal body/kinetoplast segregation in the procyclic form but by mitosis in the bloodstream form. Polo-like kinases (PLKs), known to play essential roles in regulating both mitosis and cytokinesis among eukaryotes, have a homologue in T. brucei, TbPLK, which regulates only cytokinesis. In our previous study, overexpressed triply hemagglutinin-tagged TbPLK (TbPLK-3HA) in the procyclic form localized to a mid-dorsal point and the anterior tip of the cell along the flagellum attachment zone (FAZ). In our current study, TbPLK-3HA expressed at the endogenous level was identified at the same dorsal location of both procyclic and bloodstream forms, albeit it was no longer detectable at the anterior tip of the cell. Endogenously expressed TbPLK fused with an enhanced yellow fluorescent protein (EYFP) localized to the same dorsal location along the FAZs in living procyclic and bloodstream cells. Fluorescence-activated cell sorter analysis of hydroxyurea-synchronized procyclic cells revealed that TbPLK-EYFP emerges during S phase, persists through G(2)/M phase, and vanishes in G(1) phase. An indicated TbPLK-EYFP association with the FAZs of G(2)/M cells may thus represent a timely localization to a potential initiation site of cytokinesis, which agrees with the recognized role of TbPLK in cytokinetic initiation.
Figures




References
-
- Abrieu, A., T. Brassac, S. Galas, D. Fisher, J. C. Labbe, and M. Doree. 1998. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 1111751-1757. - PubMed
-
- Alexandru, G., F. Uhlmann, K. Mechtler, M. A. Poupart, and K. Nasmyth. 2001. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105459-472. - PubMed
-
- Barr, F. A., H. H. Sillje, and E. A. Nigg. 2004. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5429-440. - PubMed
-
- Berriman, M., E. Ghedin, C. Hertz-Fowler, G. Blandin, H. Renauld, D. C. Bartholomeu, N. J. Lennard, E. Caler, N. E. Hamlin, B. Haas, U. Bohme, L. Hannick, M. A. Aslett, J. Shallom, L. Marcello, L. Hou, B. Wickstead, U. C. Alsmark, C. Arrowsmith, R. J. Atkin, A. J. Barron, F. Bringaud, K. Brooks, M. Carrington, I. Cherevach, T. J. Chillingworth, C. Churcher, L. N. Clark, C. H. Corton, A. Cronin, R. M. Davies, J. Doggett, A. Djikeng, T. Feldblyum, M. C. Field, A. Fraser, I. Goodhead, Z. Hance, D. Harper, B. R. Harris, H. Hauser, J. Hostetler, A. Ivens, K. Jagels, D. Johnson, J. Johnson, K. Jones, A. X. Kerhornou, H. Koo, N. Larke, S. Landfear, C. Larkin, V. Leech, A. Line, A. Lord, A. Macleod, P. J. Mooney, S. Moule, D. M. Martin, G. W. Morgan, K. Mungall, H. Norbertczak, D. Ormond, G. Pai, C. S. Peacock, J. Peterson, M. A. Quail, E. Rabbinowitsch, M. A. Rajandream, C. Reitter, S. L. Salzberg, M. Sanders, S. Schobel, S. Sharp, M. Simmonds, A. J. Simpson, L. Tallon, C. M. Turner, A. Tait, A. R. Tivey, S. Van Aken, D. Walker, D. Wanless, S. Wang, B. White, O. White, S. Whitehead, J. Woodward, J. Wortman, M. D. Adams, T. M. Embley, K. Gull, E. Ullu, J. D. Barry, A. H. Fairlamb, F. Opperdoes, B. G. Barrell, J. E. Donelson, N. Hall, C. M. Fraser, S. E. Melville, and N. M. El-Sayed. 2005. The genome of the African trypanosome Trypanosoma brucei. Science 309416-422. - PubMed
-
- Briggs, L. J., P. G. McKean, A. Baines, F. Moreira-Leite, J. Davidge, S. Vaughan, and K. Gull. 2004. The flagella connector of Trypanosoma brucei: an unusual mobile transmembrane junction. J. Cell Sci. 1171641-1651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

