The Omp85-related chloroplast outer envelope protein OEP80 is essential for viability in Arabidopsis
- PMID: 18621981
- PMCID: PMC2528115
- DOI: 10.1104/pp.108.122754
The Omp85-related chloroplast outer envelope protein OEP80 is essential for viability in Arabidopsis
Abstract
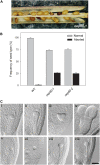
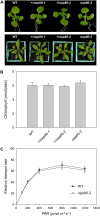
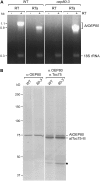
beta-Barrel proteins of the Omp85 (Outer membrane protein, 85 kD) superfamily exist in the outer membranes of Gram-negative bacteria, mitochondria, and chloroplasts. Prominent Omp85 proteins in bacteria and mitochondria mediate biogenesis of other beta-barrel proteins and are indispensable for viability. In Arabidopsis (Arabidopsis thaliana) chloroplasts, there are two distinct types of Omp85-related protein: Toc75 (Translocon at the outer envelope membrane of chloroplasts, 75 kD) and OEP80 (Outer Envelope Protein, 80 kD). Toc75 functions as a preprotein translocation channel during chloroplast import, but the role of OEP80 remains elusive. We characterized three T-DNA mutants of the Arabidopsis OEP80 (AtOEP80) gene. Selectable markers associated with the oep80-1 and oep80-2 insertions segregated abnormally, suggesting embryo lethality of the homozygous genotypes. Indeed, no homozygotes were identified among >100 individuals, and heterozygotes of both mutants produced approximately 25% aborted seeds upon self-pollination. Embryo arrest occurred at a relatively late stage (globular embryo proper) as revealed by analysis using Nomarski optics microscopy. This is substantially later than arrest caused by loss of the principal Toc75 isoform, atToc75-III (two-cell stage), suggesting a more specialized role for AtOEP80. Surprisingly, the oep80-3 T-DNA (located in exon 1 between the first and second ATG codons of the open reading frame) did not cause any detectable developmental defects or affect the size of the AtOEP80 protein in chloroplasts. This indicates that the N-terminal region of AtOEP80 is not essential for the targeting, biogenesis, or functionality of the protein, in contrast with atToc75-III, which requires a bipartite targeting sequence.
Figures


References
-
- Aronsson H, Boij P, Patel R, Wardle A, Töpel M, Jarvis P (2007) Toc64/OEP64 is not essential for the efficient import of proteins into chloroplasts in Arabidopsis thaliana. Plant J 52 53–68 - PubMed
-
- Aronsson H, Combe J, Jarvis P (2003) Unusual nucleotide-binding properties of the chloroplast protein import receptor, atToc33. FEBS Lett 544 79–85 - PubMed
-
- Aronsson H, Jarvis P (2002) A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett 529 215–220 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

