Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity
- PMID: 18622013
- PMCID: PMC2528983
- DOI: 10.1074/jbc.M804800200
Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity
Abstract
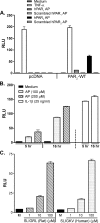
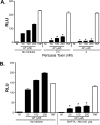
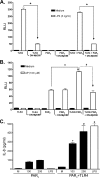
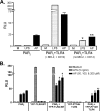
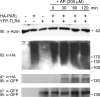
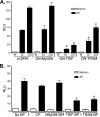
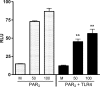
Proteinase-activated receptor 2 (PAR2), a seven-transmembrane G protein-coupled receptor, is activated at inflammatory sites by proteolytic cleavage of its extracellular N terminus by trypsin-like enzymes, exposing a tethered, receptor-activating ligand. Synthetic agonist peptides (AP) that share the tethered ligand sequence also activate PAR2, often measured by Ca2+ release. PAR2 contributes to inflammation through activation of NF-kappaB-regulated genes; however, the mechanism by which this occurs is unknown. Overexpression of human PAR2 in HEK293T cells resulted in concentration-dependent, PAR2 AP-inducible NF-kappaB reporter activation that was protein synthesis-independent, yet blocked by inhibitors that uncouple Gi proteins or sequester intracellular Ca2+. Because previous studies described synergistic PAR2- and TLR4-mediated cytokine production, we hypothesized that PAR2 and TLR4 might interact at the level of signaling. In the absence of TLR4, PAR2-induced NF-kappaB activity was inhibited by dominant negative (DN)-TRIF or DN-TRAM constructs, but not by DN-MyD88, findings confirmed using cell-permeable, adapter-specific BB loop blocking peptides. Co-expression of TLR4/MD-2/CD14 with PAR2 in HEK293T cells led to a synergistic increase in AP-induced NF-kappaB signaling that was MyD88-dependent and required a functional TLR4, despite the fact that AP exhibited no TLR4 agonist activity. Co-immunoprecipitation of PAR2 and TLR4 revealed a physical association that was AP-dependent. The response to AP or lipopolysaccharide was significantly diminished in TLR4(-/-) and PAR2(-/-) macrophages, respectively, and SW620 colonic epithelial cells exhibited synergistic responses to co-stimulation with AP and lipopolysaccharide. Our data suggest a unique interaction between two distinct innate immune response receptors and support a novel paradigm of receptor cooperativity in inflammatory responses.
Figures
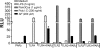

Similar articles
-
Inhibition of TLR4 signaling by TRAM-derived decoy peptides in vitro and in vivo.J Immunol. 2013 Mar 1;190(5):2263-72. doi: 10.4049/jimmunol.1202703. Epub 2013 Jan 23. J Immunol. 2013. PMID: 23345333 Free PMC article.
-
The Asp299Gly polymorphism alters TLR4 signaling by interfering with recruitment of MyD88 and TRIF.J Immunol. 2012 May 1;188(9):4506-15. doi: 10.4049/jimmunol.1200202. Epub 2012 Apr 2. J Immunol. 2012. PMID: 22474023 Free PMC article.
-
TRIF signaling is essential for TLR4-driven IgE class switching.J Immunol. 2014 Mar 15;192(6):2651-8. doi: 10.4049/jimmunol.1300909. Epub 2014 Feb 14. J Immunol. 2014. PMID: 24532577 Free PMC article.
-
Pellino-3 promotes endotoxin tolerance and acts as a negative regulator of TLR2 and TLR4 signaling.J Leukoc Biol. 2015 Dec;98(6):963-74. doi: 10.1189/jlb.2VMA0515-229RR. Epub 2015 Aug 26. J Leukoc Biol. 2015. PMID: 26310831 Free PMC article.
-
TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling.Cell Mol Life Sci. 2021 Feb;78(4):1233-1261. doi: 10.1007/s00018-020-03656-y. Epub 2020 Oct 15. Cell Mol Life Sci. 2021. PMID: 33057840 Free PMC article. Review.
Cited by
-
Downregulation of Matriptase Inhibits Porphyromonas gingivalis Lipopolysaccharide-Induced Matrix Metalloproteinase-1 and Proinflammatory Cytokines by Suppressing the TLR4/NF-κB Signaling Pathways in Human Gingival Fibroblasts.Biomed Res Int. 2022 Oct 4;2022:3865844. doi: 10.1155/2022/3865844. eCollection 2022. Biomed Res Int. 2022. PMID: 36246974 Free PMC article.
-
Danger of frustrated sensors: Role of Toll-like receptors and NOD-like receptors in aseptic and septic inflammations around total hip replacements.J Orthop Translat. 2017 Jul;10:68-85. doi: 10.1016/j.jot.2017.05.004. Epub 2017 Jun 7. J Orthop Translat. 2017. PMID: 29130033 Free PMC article.
-
Reply: Protease Plays a Role in Ragweed Pollen-Induced Neutrophil Recruitment and Epithelial Barrier Disruption.Am J Respir Cell Mol Biol. 2017 Feb;56(2):272-273. doi: 10.1165/rcmb.2016-0281LE. Am J Respir Cell Mol Biol. 2017. PMID: 28145773 Free PMC article. No abstract available.
-
Activation of the Coagulation Cascade as a Universal Danger Sign.Curr Issues Mol Biol. 2025 Feb 9;47(2):108. doi: 10.3390/cimb47020108. Curr Issues Mol Biol. 2025. PMID: 39996829 Free PMC article. Review.
-
Respiratory syncytial virus fusion protein-induced toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2.mBio. 2012 Aug 7;3(4):e00218-12. doi: 10.1128/mBio.00218-12. Print 2012. mBio. 2012. PMID: 22872782 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

